Chimeric Antigen Receptor and Its Expression Gene, Light Controlled Modulation of Chimeric Antigen Receptor Modified T Cell and Its Application
A technology of chimeric antigen receptors and gene expression, applied to genetically modified cells, cells modified by introducing foreign genetic material, receptors/cell surface antigens/cell surface determinants, etc., can solve poor specificity and side effects of treatment Waiting for questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
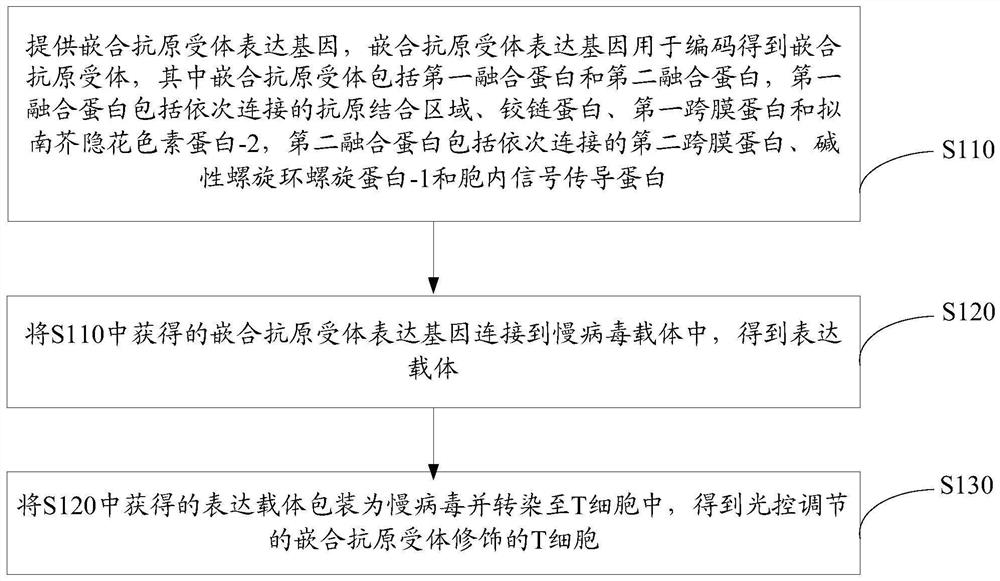
[0119] see image 3 , a method for preparing light-regulated chimeric antigen receptor-modified T cells according to one embodiment, comprising the following steps S110-S130.
[0120] S110, providing a chimeric antigen receptor expression gene, the chimeric antigen receptor expression gene is used to encode a chimeric antigen receptor, wherein the chimeric antigen receptor includes a first fusion protein and a second fusion protein, and the first fusion protein includes The antigen-binding domain, hinge protein, first transmembrane protein and Arabidopsis cryptochrome protein-2 are connected sequentially, and the second fusion protein includes the second transmembrane protein, basic helix loop helix protein-1 and cytoplasmic protein-1 connected sequentially. Internal signaling proteins.
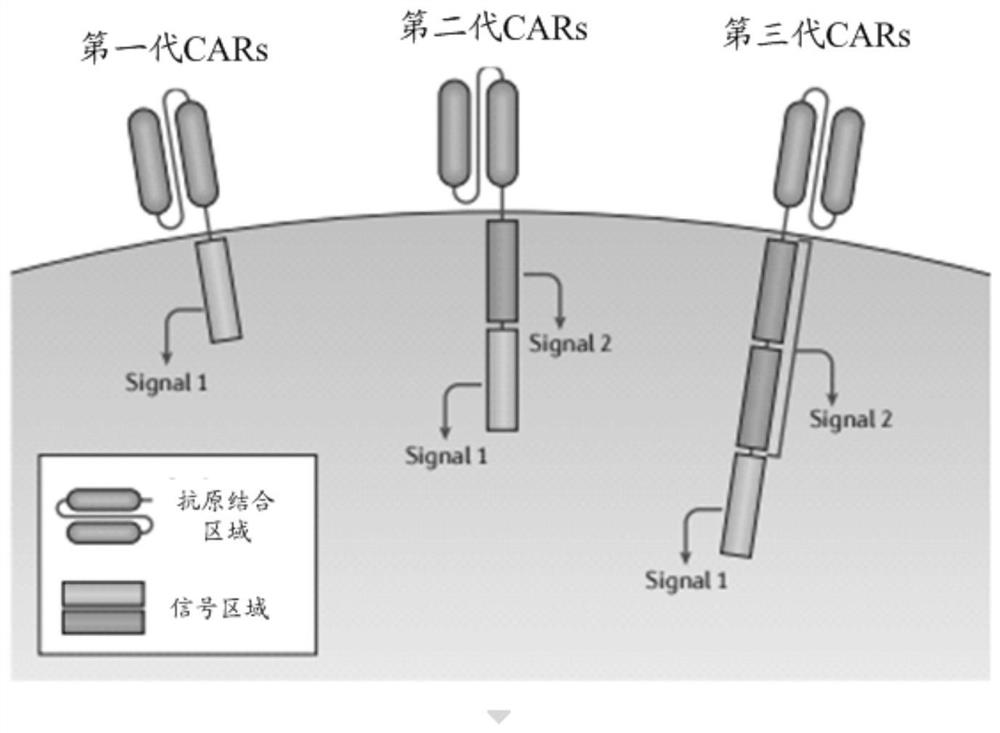
[0121] Specifically, the structure of the chimeric antigen receptor (CAR) can be referred to above, and will not be repeated here.
[0122] In one embodiment, the expression of the encoded ...
Embodiment 1
[0136] Preparation of light-regulated chimeric antigen receptor-modified T cells
[0137] Synthesize the nucleic acid sequence (comprising the nucleotide sequence shown in SEQ ID No.1, the nucleotide sequence shown in SEQ ID No.2, the nucleotide sequence shown in SEQ ID No.3 connected sequentially by the gene company) of the first fusion protein respectively The nucleotide sequence and the nucleotide sequence shown in SEQ ID No.4). The expression of the first fusion protein is anti-mesothelin-CD8αhinge-CD28TM-CD28-4-1BB-CRY2.
[0138] Synthesize the nucleic acid sequence (comprising the nucleotide sequence shown in SEQ ID No.5, the nucleotide sequence shown in SEQ ID No.6 and the nucleotide sequence shown in SEQ ID No.7 connected sequentially by the gene company respectively) of encoding the second fusion protein nucleotide sequence). The expression of the second fusion protein is CD28-4-1BB-CIB1-CD3ζ.
[0139] The nucleic acid sequence encoding the first fusion protein and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com