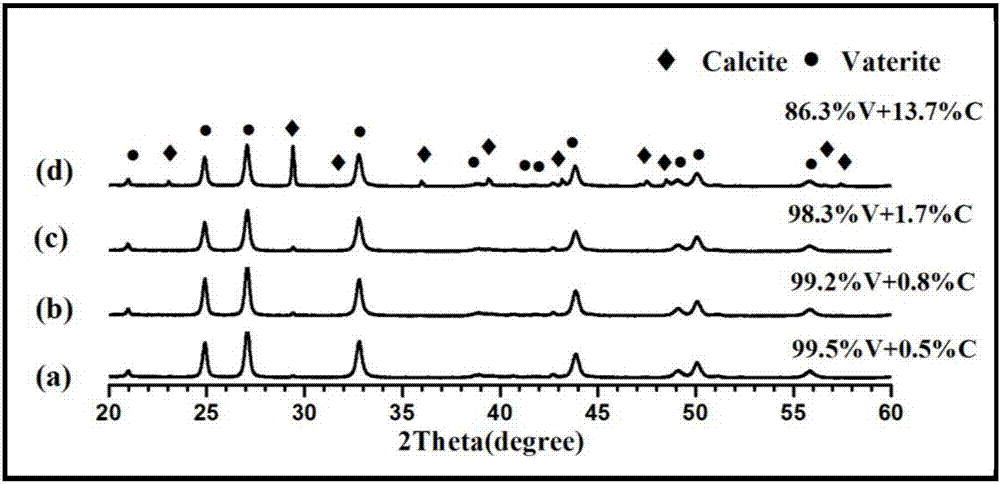
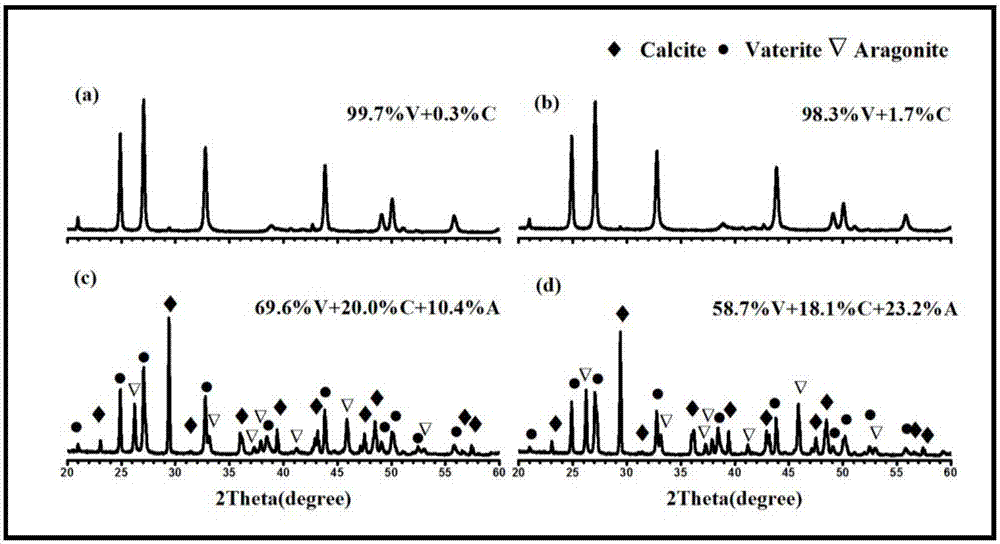
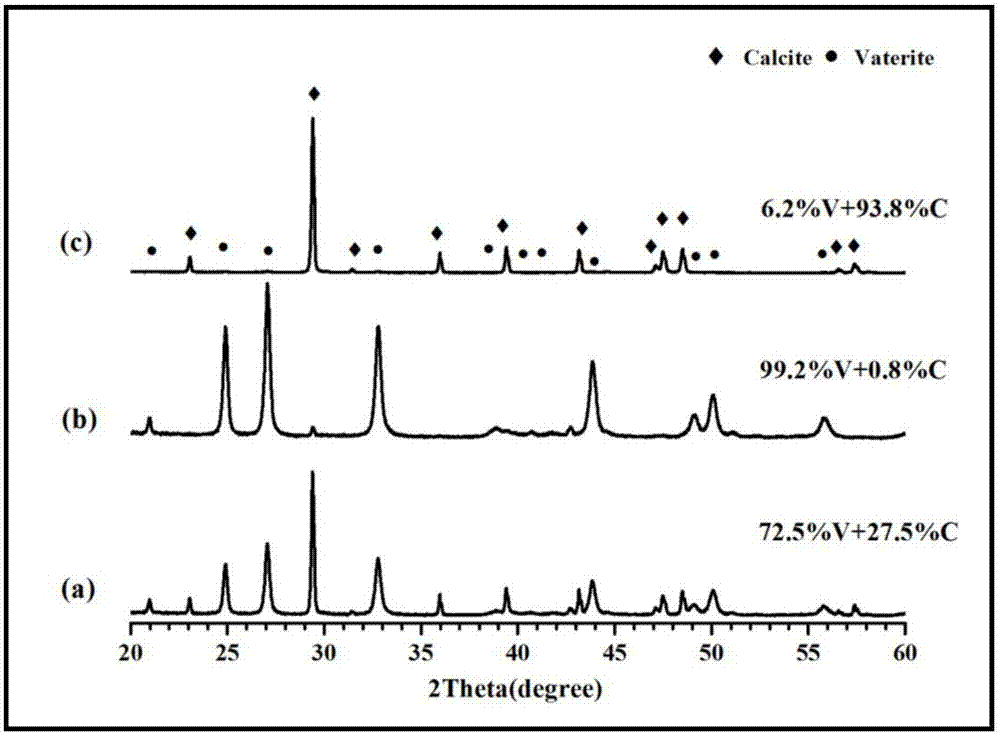
Method for preparing metastable-state vaterite calcium carbonate based on calciumethylene glycolate calcium method
A technology of calcium ethylene glycol and ethylene glycol, applied in the direction of calcium carbonate/strontium/barium, etc., to achieve the effects of simple process conditions, low cost of raw materials and equipment, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1) 1.11g CaCl 2 Completely dissolved in 25mL of ethylene glycol to configure a calcium glycol solution with a concentration of 0.4mol / L; 1.06g Na 2 CO 3 Completely dissolve in 10mL deionized water to prepare a sodium carbonate solution with a concentration of 1mol / L;
[0045] 2) Mix the calcium ethylene glycol solution and the aqueous sodium carbonate solution in step 1) at room temperature (20°C), stir for 2 minutes to allow them to fully react, and do not age at room temperature (20°C), that is, age for 0 min;
[0046] 3) Centrifuge the unaged mixture suspension in step 2), then wash with absolute ethanol twice and wash with deionized water twice, wash with absolute ethanol to remove calcium chloride, wash with deionized water to remove sodium carbonate and sodium chloride, centrifuge, filter, collect the obtained white solid, and dry it in an electric constant temperature blast drying oven for 5-12 hours, and finally the white powder is stored in a centrifuge tube ...
Embodiment 2
[0048] 1) 1.11g CaCl 2 Completely dissolve in 25mL of ethylene glycol to form a calcium glycol solution with a concentration of 0.4mol / L; 1.24g Na 2 CO 3 ·H 2 O was completely dissolved in 9.82mL deionized water to form a sodium carbonate solution with a concentration of 1mol / L;
[0049] 2) Mix the calcium ethylene glycol solution and sodium carbonate aqueous solution in step 1) at room temperature (20°C), stir for 2 minutes to allow them to fully react, and then age at room temperature (20°C) for 10 minutes;
[0050] 3) When the aging time is terminated, the mixture suspension in step 2) is centrifuged, then washed with absolute ethanol twice and washed with deionized water twice, the calcium chloride is removed by washing with absolute ethanol, and the calcium chloride is removed by washing with deionized water Sodium carbonate and sodium chloride were centrifuged, filtered, and the obtained white solid was collected and dried in an electric constant temperature blast dry...
Embodiment 3
[0052] 1) 1.11g CaCl 2 Completely dissolved in 25mL of ethylene glycol to configure a calcium glycol solution with a concentration of 0.4mol / L; 1.06g Na 2 CO 3 Completely dissolve in 10mL deionized water to prepare a sodium carbonate solution with a concentration of 1mol / L;
[0053] 2) Mix the calcium ethylene glycol solution and sodium carbonate aqueous solution in step 1) at room temperature (20°C), stir for 2 minutes to allow them to fully react, and then age at room temperature (20°C) for 60 minutes;
[0054] 3) When the aging time is terminated, the mixture suspension in step 2) is centrifuged, then washed with absolute ethanol twice and washed with deionized water twice, the calcium chloride is removed by washing with absolute ethanol, and the calcium chloride is removed by washing with deionized water Sodium carbonate and sodium chloride were centrifuged, filtered, and the obtained white solid was collected and dried in an electric constant temperature blast drying ov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com