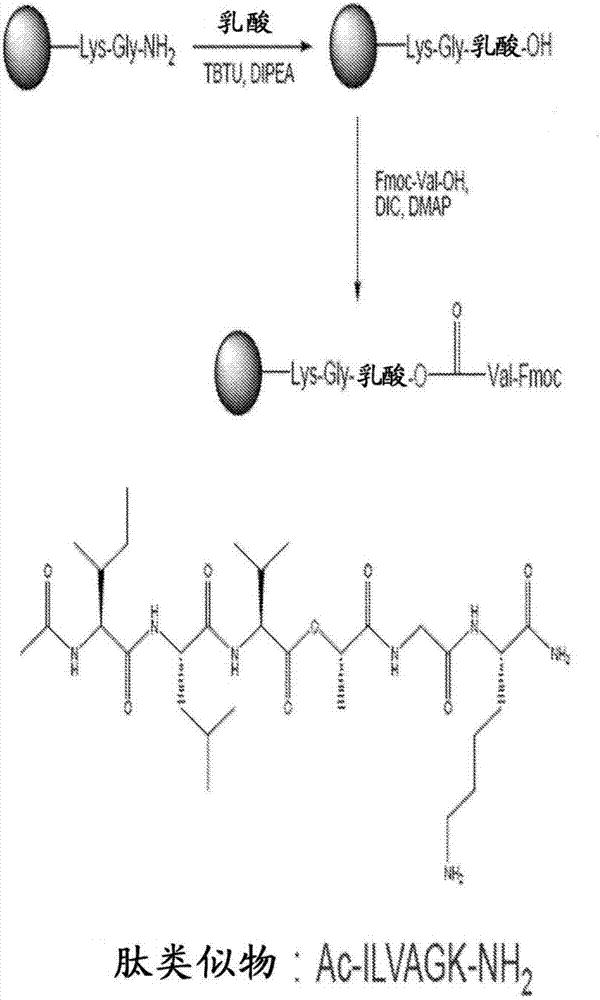
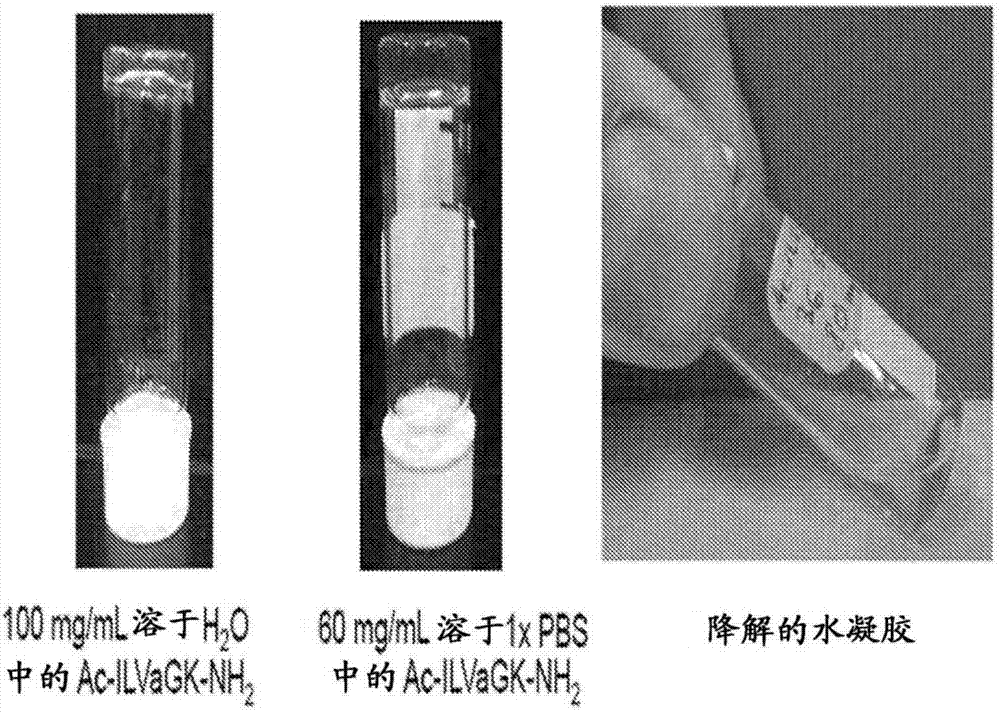
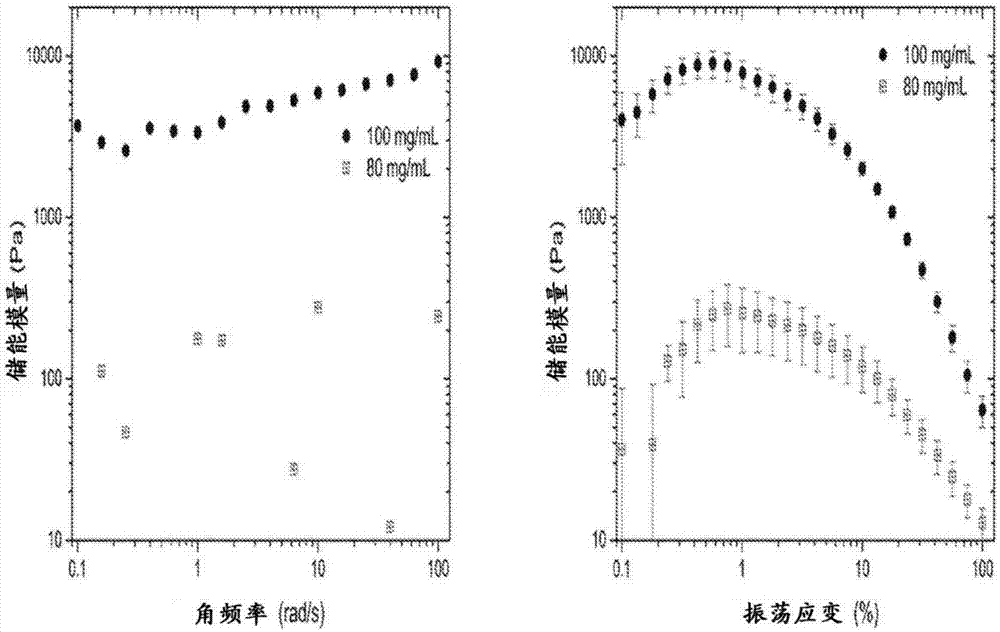
Self-assembling ultrashort aliphatic depsipeptides for biomedical applications
A technology for self-assembling, aliphatic amino acids with applications in cell and tissue engineering and nanomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0246] 1. Materials and Methods
[0247] 1.1 Materials
[0248] Fmoc-lys-rink resin (0.42 mg / mol resin), Fmoc-protected amino acids, namely glycine, alanine, valine, leucine and isoleucine, 2-(7-aza-1H-phenyl Triazole)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) was purchased from GL Biochem (Shanghai) Co., Ltd. Dimethylformamide (DMF) (analytical grade) was purchased from Fisher Scientific UK. Acetic anhydride (Ac 2 O) and dimethylsulfoxide (DMSO) were purchased from Sigma Aldrich. N,N-Diisopropylethylamine (DIPEA), dichloromethane (DCM), trifluoroacetic acid (TFA) and TIS (triisopropylsilane) were purchased from Alfa Aesar, a Johnson Matthey Company. Piperidine was purchased from Merck Schuchardt OHG. Diethyl ether (Et 2 O) was purchased from Tedia Company Inc. and lactic acid was purchased from Sigma-Aldrich. All chemicals were used as received.
[0249] All peptide-based compounds were purified on an Agilent 1260 Infinity preparative HPLC system equipped ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com