Smart glutathione sulfur transferase based on light-responsive supramolecular switch
A glutathione and sulfur transfer technology, applied in the field of biomedicine, can solve the problems of narrow scope of application, poor versatility, low efficiency of catalyst molecules, etc., and achieve the effects of simple design, wide practicability, and good enzymatic activity and regulation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
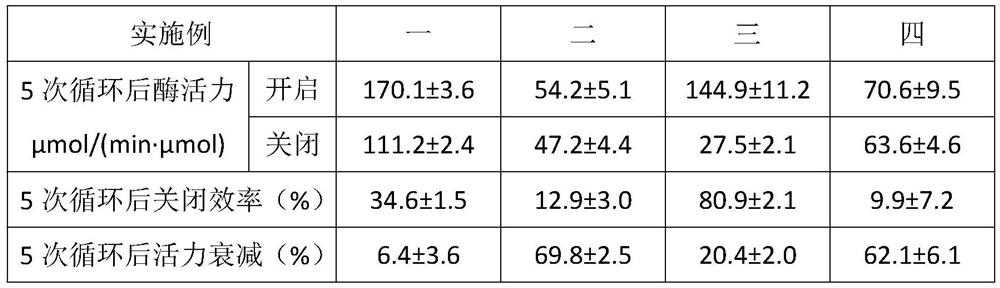
Examples
Embodiment 1
[0017] The preparation method of the intelligent glutathione sulfur transferase based on light-responsive supramolecular switch construction proposed by the present invention comprises the following steps:
[0018] S1. Perform site-directed mutagenesis on GST: identify the catalytic site of GST. The catalytic site is located at the bottom of the GSH binding cavity of the GST substrate and contains several key amino acid residues. The key amino acid residues are W7, W40, K44, N53, P55, Q66, S67, and D100, replace C84S, C137S, C168H, and C177S on the GST with serine or histidine, and then introduce the K124C mutation to the outer edge of the GSH-binding cavity of the GST, using Specific modification on GST;
[0019] S2. Preparation of trans-Azo-MAM-CD complex: non-covalent compounding of α-CD and maleimide-modified azobenzene derivatives to construct a photoresponsive supramolecular switch, while using one-dimensional nuclear magnetic resonance hydrogen Spectra, ESI mass spectr...
Embodiment 2
[0022] The preparation method of the intelligent glutathione sulfur transferase based on light-responsive supramolecular switch construction proposed by the present invention comprises the following steps:
[0023] S1. Perform site-directed mutagenesis on GST: identify the catalytic site of GST. The catalytic site is located at the bottom of the GSH binding cavity of the GST substrate and contains several key amino acid residues. The key amino acid residues are W7, W40, K44, N53, P55, Q66, S67, and D100, replace C84S, C137S, C168H, and C177S on the GST with serine or histidine, and then introduce the K124C mutation to the outer edge of the GSH-binding cavity of the GST, using Specific modification on GST;
[0024] S2. Preparation of trans-Azo-MAM-CD complex: β-CD and maleimide-modified azobenzene derivatives were non-covalently compounded to construct a photoresponsive supramolecular switch. At the same time, one-dimensional nuclear magnetic resonance was used The trans-Azo-M...
Embodiment 3
[0027] The preparation method of the intelligent glutathione sulfur transferase based on light-responsive supramolecular switch construction proposed by the present invention comprises the following steps:
[0028] S1. Perform site-directed mutagenesis on GST: identify the catalytic site of GST. The catalytic site is located at the bottom of the GSH binding cavity of the GST substrate and contains several key amino acid residues. The key amino acid residues are W7, W40, K44, N53, P55, Q66, S67, and D100, replace C84S, C137S, C168H, and C177S on GST with serine or histidine, and then introduce two mutations, L117C and K124C, into the GSH binding cavity of GST outer edge, for specific modification of GST;
[0029] S2. Preparation of trans-Azo-MAM-CD complex: non-covalent compounding of α-CD and maleimide-modified azobenzene derivatives to construct a photoresponsive supramolecular switch, while using one-dimensional nuclear magnetic resonance hydrogen Spectra, ESI mass spectrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com