New crystal form and preparation method of cefotiam hydrochloride
A technology of cefotiam hydrochloride and crystal form, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve the problem of solvent residue, etc., and achieve the effects of low organic solvent residue, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
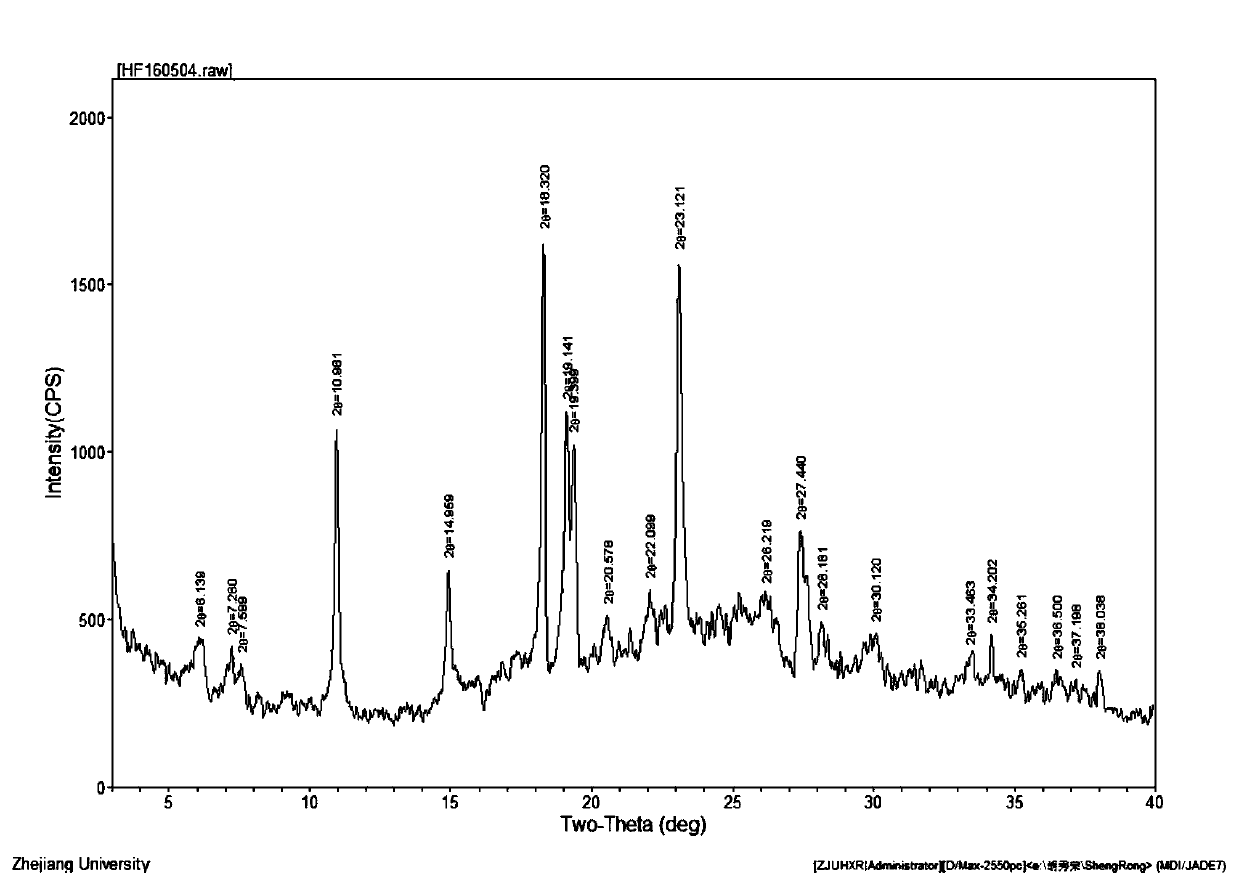
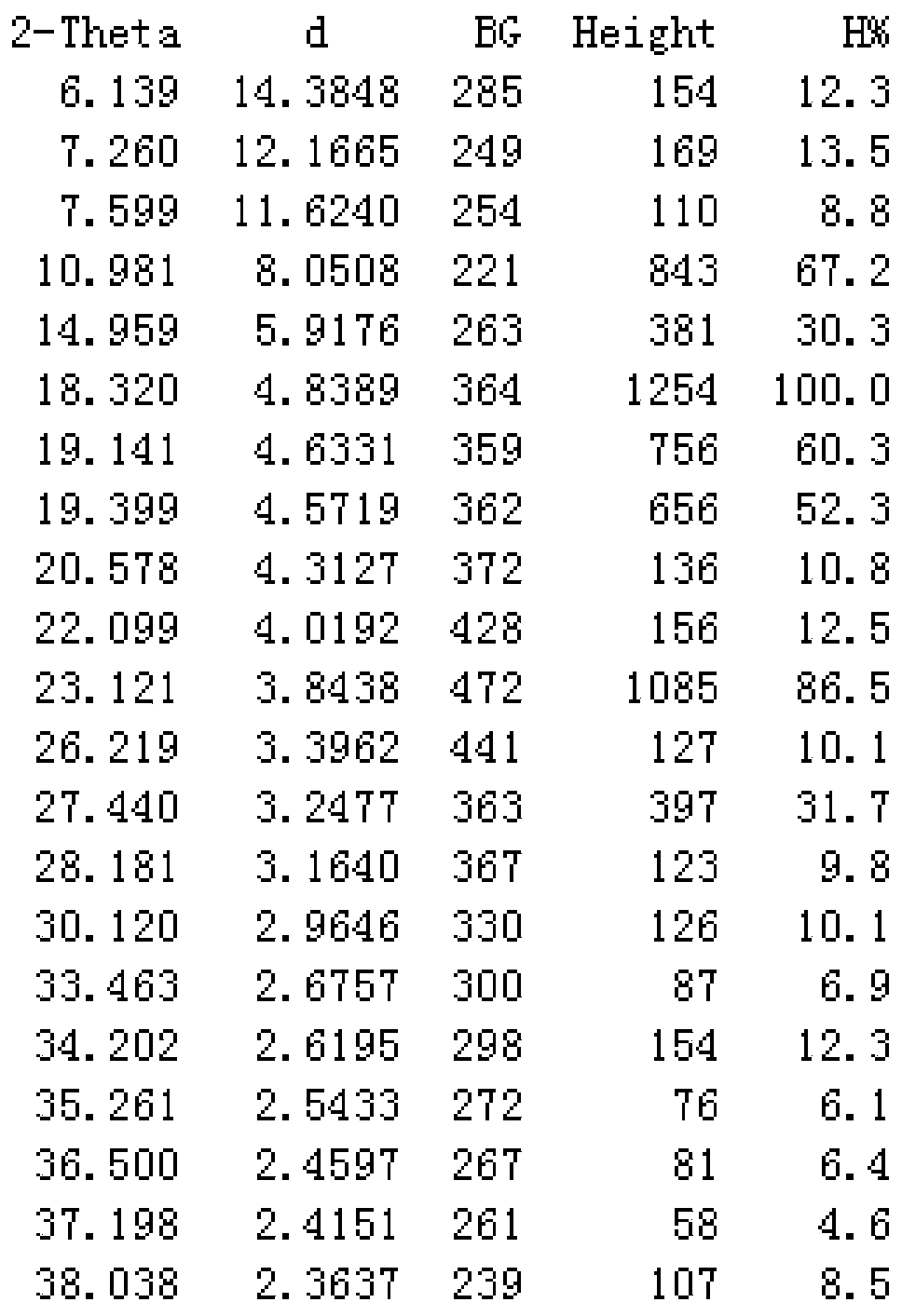
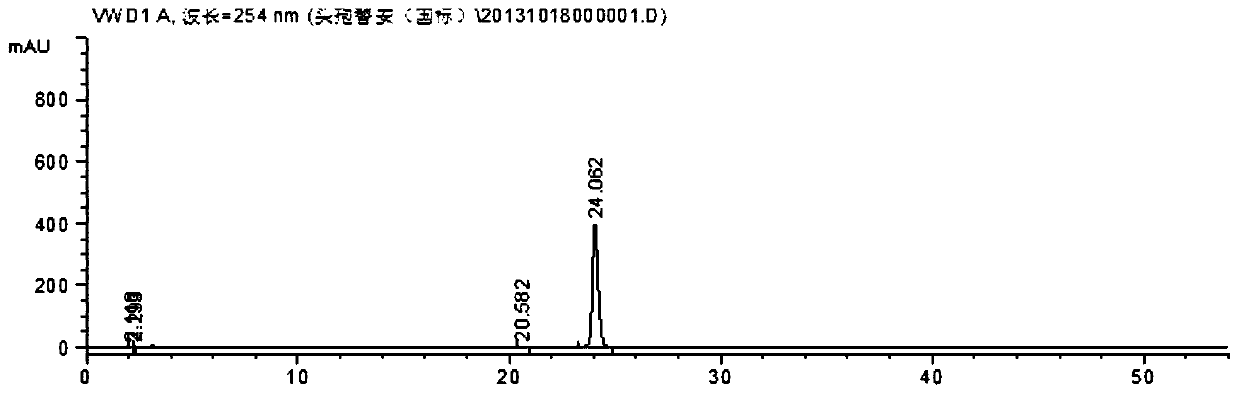
[0024] In a flask, dissolve 562g (1mol) of crude cefotiam hydrochloride in 1000mL of water, add 150mL of concentrated hydrochloric acid, 4000mL of ethanol, and 0.5mol of polyvinylpyrrolidone in sequence, heat to dissolve with microwave at 50°C, and slowly cool down to 25°C to stir and crystallize After 2 hours, filter, wash the filter cake twice with 200 mL of ethanol each, and dry it under vacuum at 45°C for 3 hours to obtain 545 g of cefotiam hydrochloride crystal form compound, with a yield of 97%.
Embodiment 2
[0026] In a flask, dissolve 562g (1mol) of crude cefotiam hydrochloride in 1000mL of methanol, sequentially add 150mL of concentrated hydrochloric acid, 4000mL of ethanol, and 0.5mol of polyvinylpyrrolidone, heat to dissolve with microwave at 50°C, slowly cool down to 25°C and stir to analyze After crystallizing for 2 hours, filtering, washing the filter cake twice with 200 mL of ethanol each, and vacuum drying at 45° C. for 3 hours, 96 g of cefotiam hydrochloride crystal form compound was obtained, with a yield of 17%.
Embodiment 3
[0028] In a flask, dissolve 562g (1mol) of crude cefotiam hydrochloride in 1000mL of water, add 150mL of concentrated sulfuric acid, 4000mL of ethanol, and 0.5mol of polyvinylpyrrolidone in sequence, heat to dissolve with microwave at 50°C, and slowly cool down to 25°C to stir and crystallize After 2 hours, filter, wash the filter cake twice with 200 mL of ethanol each, and dry it under vacuum at 45°C for 3 hours to obtain 39 g of cefotiam hydrochloride crystal form compound, with a yield of 7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com