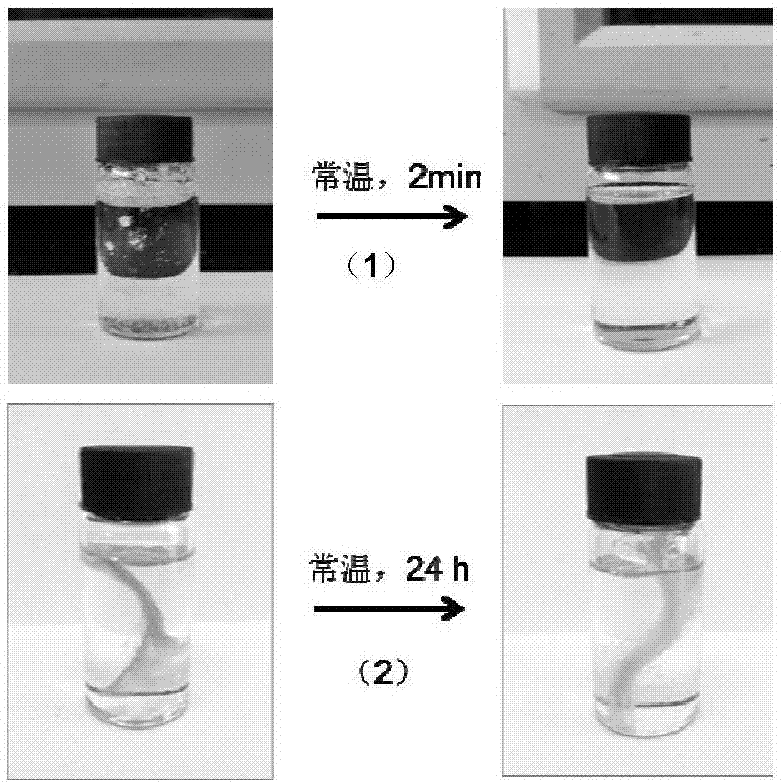
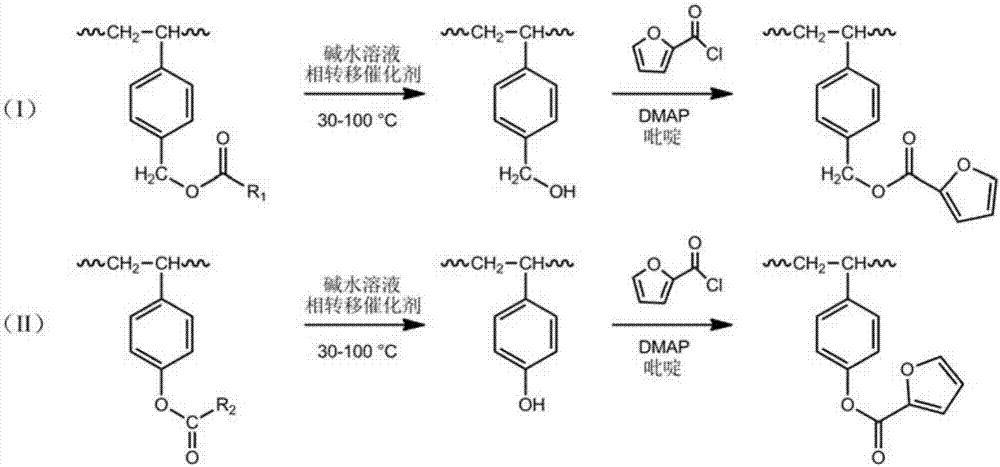
Styrene copolymers with reversible cross bonds and preparation method of styrene copolymers
A technology of cross-linked copolymer and styrene, applied in the field of styrene copolymer and its preparation, can solve the problem of losing secondary molding and the like, achieve good solvent resistance and overcome the effect of no secondary processing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Preparation of hydroxylated styrene-ethylene / butylene-styrene linear block copolymers by anionic polymerization
[0043]Vacuumize the 1L polymerization kettle, replace it with high-purity nitrogen three times, and add 650ml of purified cyclohexane first, with a designed solid content of 10%. According to table 1 and table 2 proportioning feed, first add p-4-vinylbenzyl acetate and styrene mixed monomer, 0.25ml tetrahydrofuran of the first section, start stirring, then add n-butyllithium solution according to table 2 ( Concentration 0.5M), react at 40°C for 1 hour, then add butadiene, continue to react for 1 hour, and finally add the third stage of 4-vinylbenzyl acetate and styrene mixed monomer, and react for another 1 hour. After the reaction is over, the polymerization liquid is pressed into the hydrogenation tank, and hydrogen gas is introduced to terminate the reaction. Half an hour later, add 1 mL of methyl o-toluate with a concentration of 0.02 mol / L, 30 mg o...
Embodiment 2
[0057] (1) Preparation of hydroxyl-containing star-shaped styrene-ethylene / butylene-styrene block copolymers by anionic polymerization
[0058] According to the prescription of 1-4, 1-5, 1-6 and 1-7 in table 1, synthesize the linear styrene-butadiene-styrene linear block copolymer containing 4-vinylbenzyl acetate structural unit , after adding the third-stage reaction monomer and reacting for 1 hour, press the material into the hydrogenation tank, add the coupling agent according to the formula in Table 4, and continue the reaction at 40°C for 30 minutes to obtain the structure containing 4-vinylbenzyl acetate Unit star styrene-butadiene-styrene block copolymer glue.
[0059] After the reaction was completed, hydrogenation was performed according to the method of Example 1, and the product was precipitated and dried to obtain a star-shaped styrene-ethylene / butylene-styrene block copolymer glue with 4-vinylbenzyl acetate structural units.
[0060] Dissolve the product in n-oct...
Embodiment 3
[0070] (1) Preparation of hydroxylated styrene-ethylene / propylene-styrene block copolymers by anionic polymerization
[0071] Vacuumize the 1L polymerization kettle and replace it with high-purity nitrogen three times. First add 650ml of purified cyclohexane with a designed solid content of 15%, and then feed according to the formula in Table 6 and Table 7. First add the first stage of 4-acetoxystyrene and styrene mixed monomer, 1g tetrahydrofurfuryl alcohol ethyl ether, start stirring, then add tert-butyllithium solution (concentration 0.5M), react at 60°C for 50min, Then the temperature was raised to 70° C. and isoprene was added, and the reaction was continued for 1 hour. Finally, the third stage of p-4-acetoxystyrene and styrene mixed monomer was added, and the reaction was continued for 1 hour. After the reaction is over, the polymerization liquid is pressed into the hydrogenation tank, and hydrogen gas is introduced to terminate the reaction. After half an hour, 30mg of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap