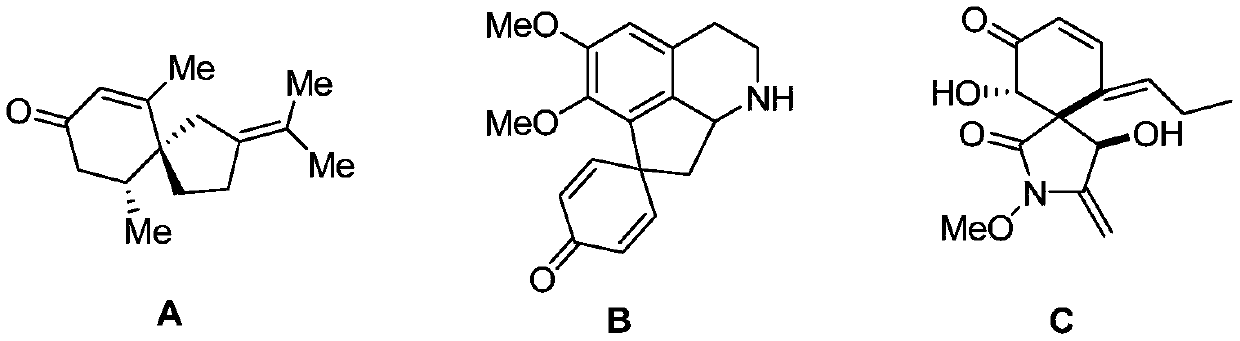
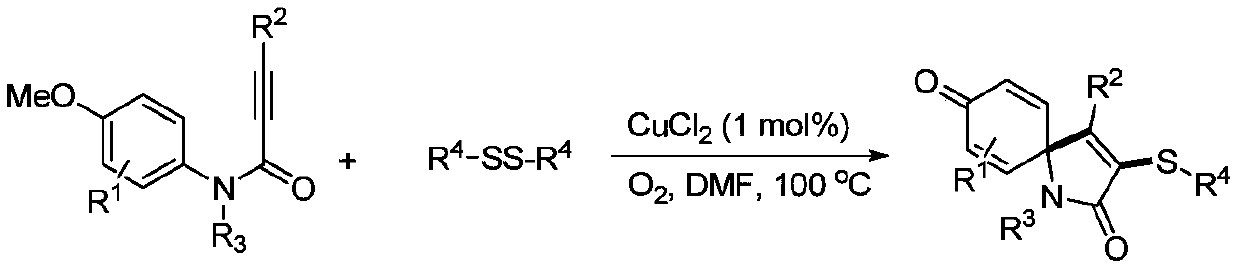
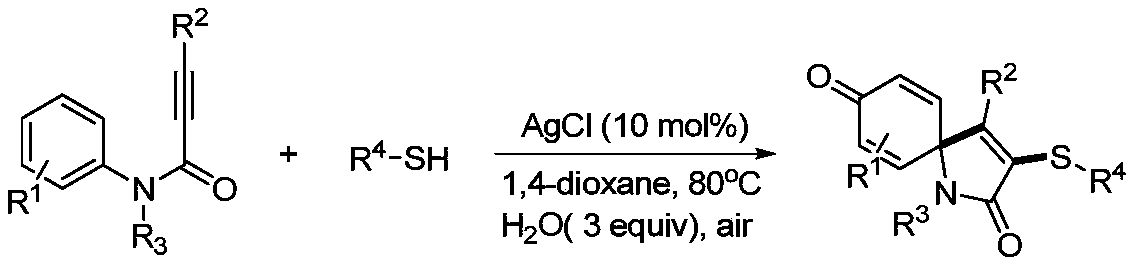A kind of preparation method of visible light-induced 3-thiospirotrienone compound
A technology of thiospirotrienone and compounds, which is applied in the field of organic synthetic chemistry, can solve the problems of harsh reaction conditions, many by-products, and high energy consumption, and achieve the effects of mild reaction conditions, simple operation, and clean energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] At room temperature, sequentially add 33.1 mg of N-(4-methoxyphenyl)-N-methyl-3-phenylpropynylamide and 31.2 mg of p-methylthiophenol into a 15 mL reaction tube, dissolve the photocatalyst in alcohol 0.8mg of eosin and 2ml of acetonitrile were mixed uniformly, then stirred and reacted for 12h under the irradiation of a 3w blue LED lamp, and after the completion of the reaction was detected by TLC, the crude product was obtained by vacuum (0.08Mpa) concentration to no solvent, and then Rinse with a mixed eluent of petroleum ether and ethyl acetate with a volume ratio of 3:1, and perform flash column chromatography on a silica gel column to obtain the 3-thiospirotrienone product of this embodiment, which is 40.6 mg of a yellow solid. The rate is 87%.
[0026] 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.31-7.28(m,1H),7.25-7.20(m,2H),7.23-7.19(m,4H),6.99(d,J=8.0Hz,2H),6.51(d ,J=10.2Hz,2H),6.45(d,J=10.3Hz,2H),2.88(s,3H),2.26(s,3H); 13 C NMR (CDCl 3 ,125MHz,ppm):δ184.0,1...
Embodiment 2
[0028]
Embodiment 3
[0032]
[0033] At room temperature, 36.9 mg of N,3-bis(4-methoxyphenyl)-N-methylphenylpropynamide, 31.2 mg of p-methylthiophenol, alcohol-soluble eosin 0.8mg, 2ml of acetonitrile solvent, mixed evenly, then stirred and reacted for 12h under the irradiation of a 3w blue LED lamp, and after the reaction was detected by TLC, it was concentrated to no solvent by vacuum (0.08Mpa) under reduced pressure to obtain a crude product, and then used Flushing with a mixed eluent of petroleum ether and ethyl acetate at a volume ratio of 2:1, followed by rapid column chromatography on a silica gel column, the 3-thiospirotrienone product of this example was obtained as 32.8 mg of a yellow oily solid, producing rate of 65%.
[0034] 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.28(t,J=8.6Hz,2H),7.21(d,J=7.9Hz,2H),7.02(d,J=7.8Hz,2H),6.78(d,J= 8.6Hz, 2H), 6.51(d, J=10.2Hz, 2H), 6.48(d, J=10.2Hz, 2H), 3.78(s, 3H), 2.86(s, 3H), 2.28(s, 3H) ; 13 C NMR (CDCl 3 ,125MHz,ppm):δ184.1,167.9,160.7,152.2,145.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com