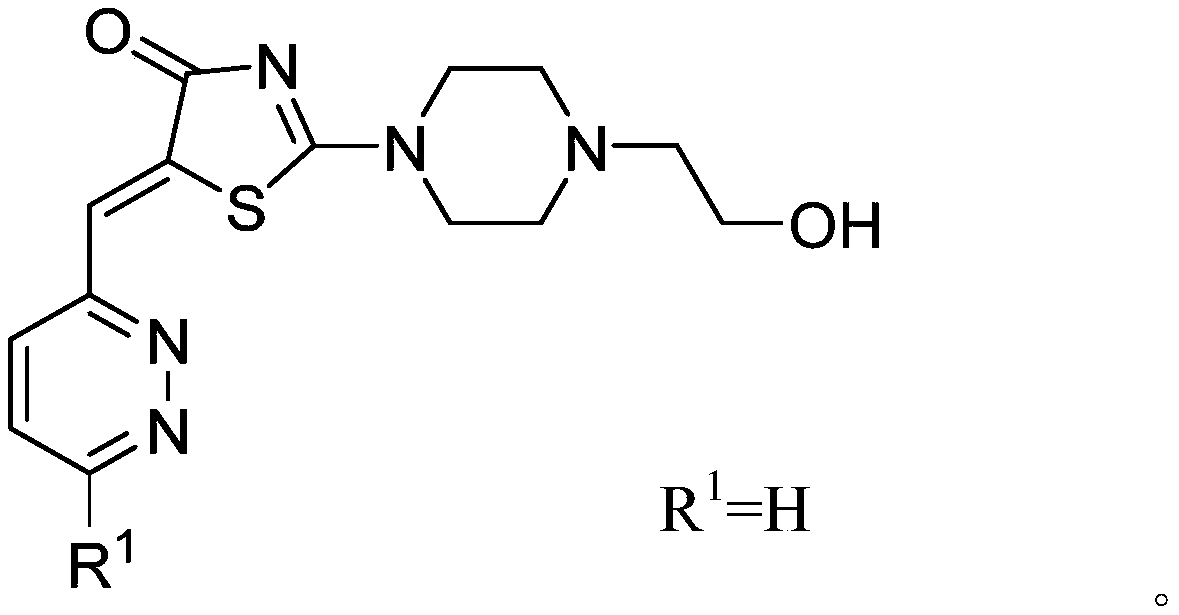
A kind of puma inhibitor and its preparation method and use
A technology of inhibitors and uses, applied in the field of medicine, to achieve the effect of blocking apoptosis, good physical and chemical properties, and good resistance to apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0056]
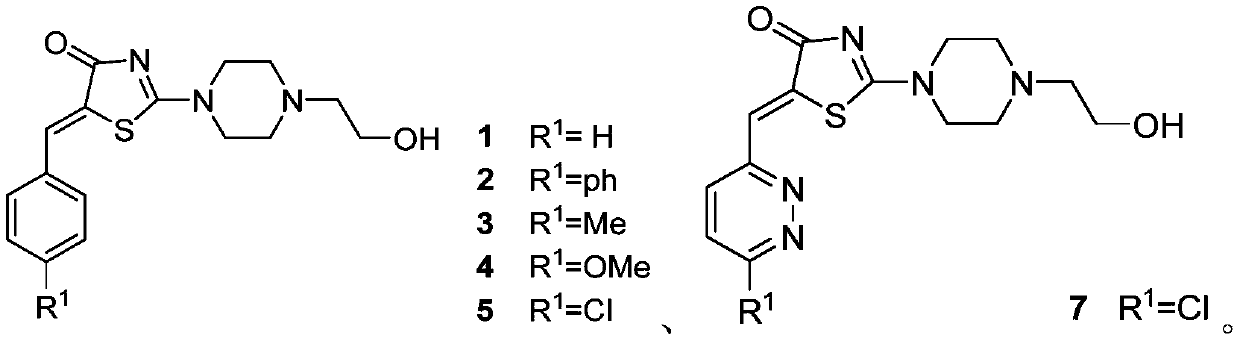
[0057] Add 2.0 g (15 mmol) of rhodanine, 20 mL of anhydrous methanol, and 0.1 mL of glacial acetic acid in a three-necked flask. 2 Under protection, 2.9 g (22.6 mmol) of hydroxyethylpiperazine and 22.6 mmol of benzaldehyde were added, and the oil bath was refluxed to obtain a light yellow clear reaction liquid, which was monitored by TLC in time. After 1-2 hours, the reaction was completed, and a yellow solid was precipitated in an ice bath. Suction filtration, washing with methanol, the crude product was purified by silica gel column chromatography (DCM:MeOH 10:1) to obtain pure compound 1 with a yield of 35-55%.
[0058] Yellow solid, 46% yield. 1 H NMR (400MHz, CDCl 3 )δ=7.83(s,1H),δ=7.56(d,2H),δ=7.47(m,2H),δ=7.41(t,1H),δ=4.1(t,2H),δ=3.69( m, 4H), δ=2.72(t, 2H), δ=2.66(m, 4H). 13 CNMR (400MHz, CDCl 3 ) 180.84, 175.22, 134.16, 131.94, 129.78, 129.74, 129.03, 127.88, 59.26, 58.05, 52.53, 52.11, 48.68, 48.34, HRMS (ESI) calcd.for C 16 h 19 N 3 o 2 S:317.1[M...
preparation Embodiment 2
[0060] Compound 2 was prepared in the same manner as in Preparation Example 1 except that p-phenylbenzaldehyde was used instead of benzaldehyde.
[0061] Pale yellow solid, yield = 52.9%, 1 H NMR (400MHz, CDCl 3 )δ=7.87(s,1H),δ=7.69(d,2H),δ=7.63(m,4H),δ=7.48(t,2H),δ=7.42(t,1H),δ=5.32( s,1H),δ=4.1(t,2H),δ=3.71(m,4H),δ=2.73(t,2H),δ=2.68(m,4H)HRMS(ESI)calcd.for C 22 h 23 N 3 o 2 S 394.2[M+H] + ,found 394.2[M+H] + .
preparation Embodiment 3
[0063] Compound 3 was prepared in a similar manner to Preparation Example 1 except that p-tolualdehyde was used instead of benzaldehyde.
[0064] Pale yellow solid, yield = 49.1%, 1 H NMR (400MHz, CDCl 3 )δ=7.81(s,1H),δ=7.43(d,2H),δ=7.27(t,3H),δ=4.12(t,2H),δ=3.70(m,4H),δ=2.73( t,2H), δ=2.68(m,4H), δ=2.40(s,3H)HRMS(ESI)calcd.for C 17 h 21 N 3 o 2 S 331.4[M+H] + ,found 331.8[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com