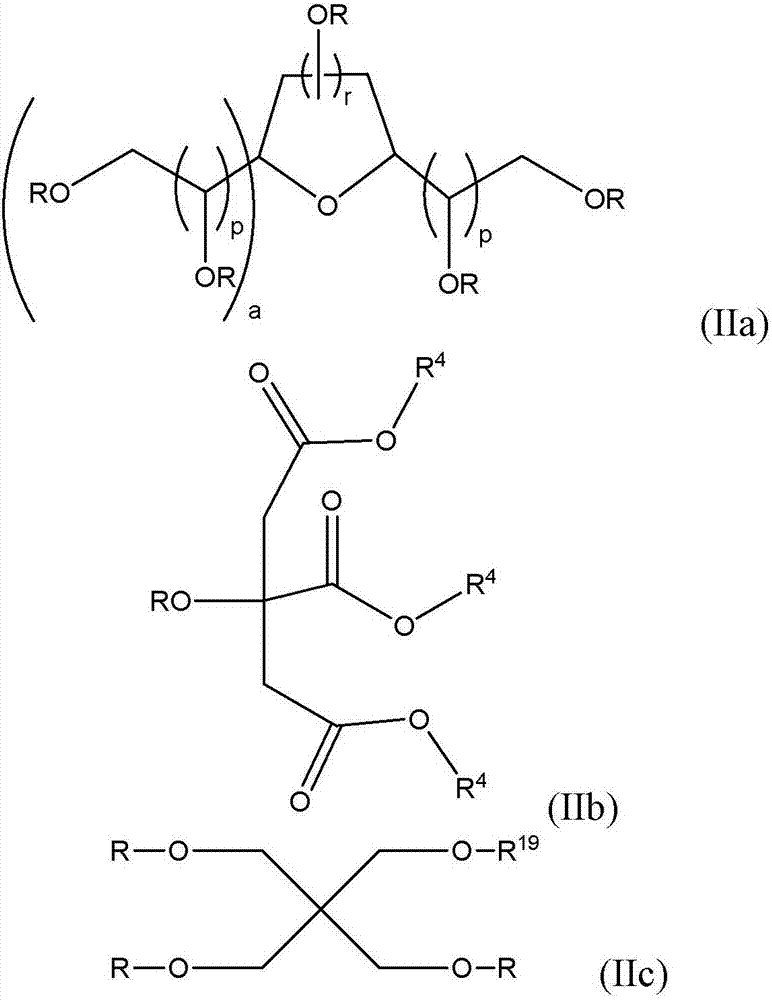
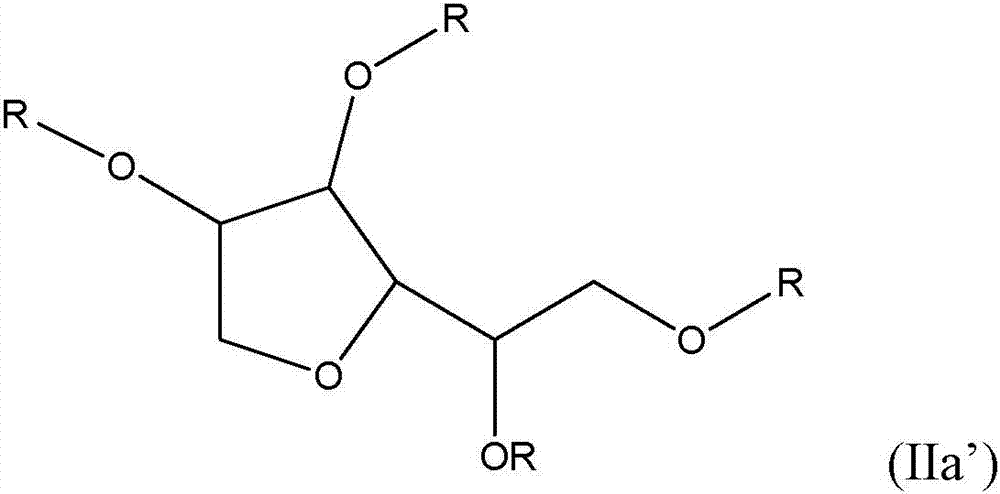
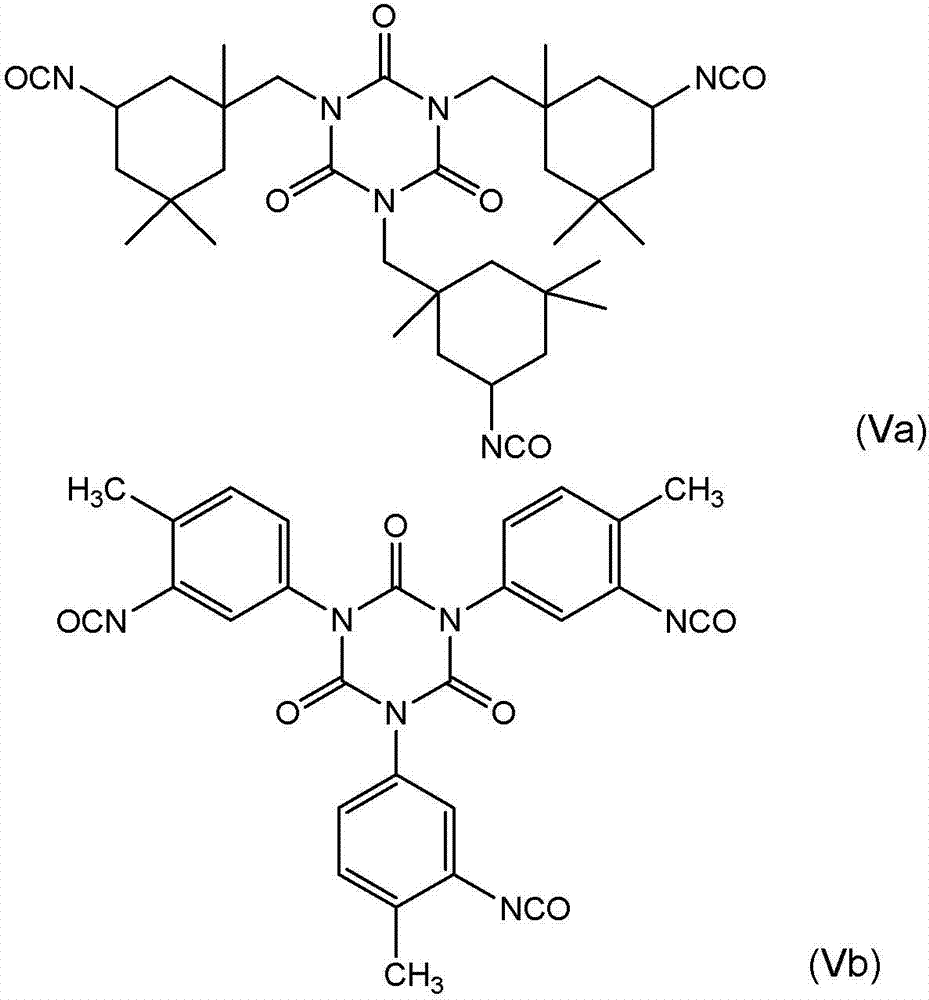
Non-fluorinated urethane based coatings
A technology of residues and linking groups, applied in the field of water-based compositions, can solve the problem of low effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0124] Preparation of trioctadecyl citrate
[0125] In a 4 necked round bottom flask equipped with overhead stirrer, thermocouple, dean-stark / condenser, stearyl alcohol (100.0 g), citric acid (20 g), toluene (150 g) and sulfuric acid (2 g) were added. The solution was refluxed for 8 hours in order to remove the water generated during the esterification. After 8 hours, the crude citrate was precipitated at 0 °C using ethanol, filtered and recrystallized.
[0126] Preparation of inositol laurate
[0127] In a 4 necked round bottom flask equipped with overhead stirrer, thermocouple, Dean-Stark trap and condenser, add inositol (15.0 g), lauric acid (75.05 g), toluene (90.05 g), p-toluenesulfonate acid (about 2g) and sulfuric acid (0.5g). The solution was heated to about 120°C and refluxed to remove water from the reactants. Once all water was removed, the reaction was cooled to 70°C and 10% by weight sodium hydroxide solution (13.0 g) was added. The reaction was stirred f...
example 1
[0147] In a 4-neck round bottom flask equipped with overhead stirrer, thermocouple, dean-stark / condenser, add sorbitol tristearate (116.0 g, hydroxyl number = 77.2 mgKOH / g) and 4-formaldehyde Amyl-2-pentanone (MIBK, 150 g). The solution was refluxed for 1 hour to remove any residual moisture. After this hour, the solution was cooled to 50°C, DESMODUR N-100 (30 g) was added, followed by the catalyst, and the solution was heated to 80°C over one hour.
example 2
[0149] Aqueous dispersions of compounds as described in Example 1 were prepared. Water (300 g), ARMEEN DM-18D (5.6 g), TERGITOL TMN-10 (2.8 g), and acetic acid (3.4 g) were added to the beaker and stirred to form a surfactant solution. The solution was heated to 60°C. The sorbitol carbamate / MIBK solution prepared as in Example 1 was cooled to 60°C and the surfactant solution was added slowly to produce a milky emulsion. The mixture was homogenized at 6000 psi, and the resulting emulsion was distilled under reduced pressure to remove solvent, resulting in a non-flammable urethane dispersion at 25% solids. The urethane dispersions were applied to textiles and tested according to the test methods described above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com