Preparation method of aluminum phosphate-coated carbonyl iron anti-oxidation wave-absorbing material
A technology for coating carbonyl iron and absorbing materials with aluminum phosphate, applied in carbonyl iron, chemical instruments and methods, phosphorus compounds, etc. Poor matching and other problems, to achieve the effect of improving electromagnetic parameters, improving thermal stability, and good oxidation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
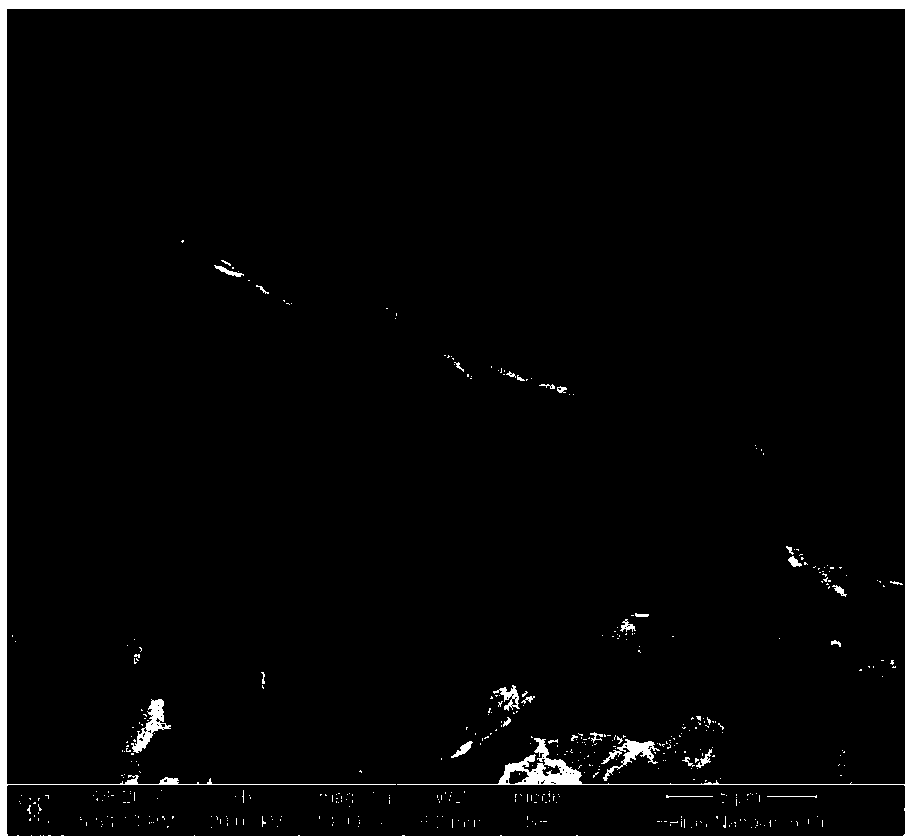
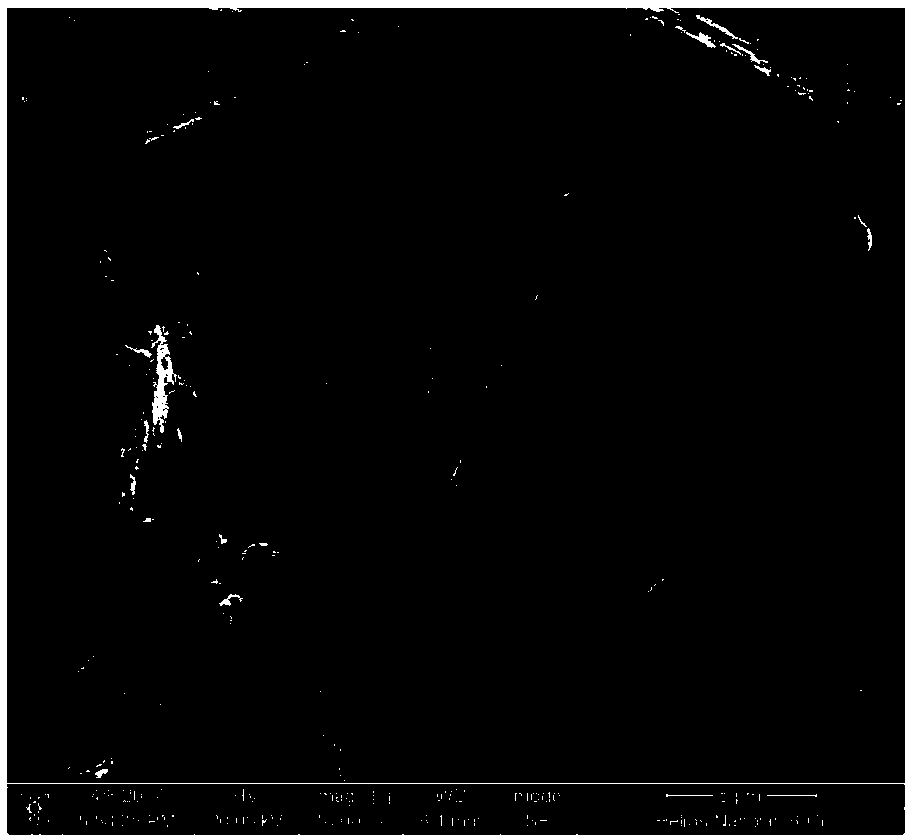
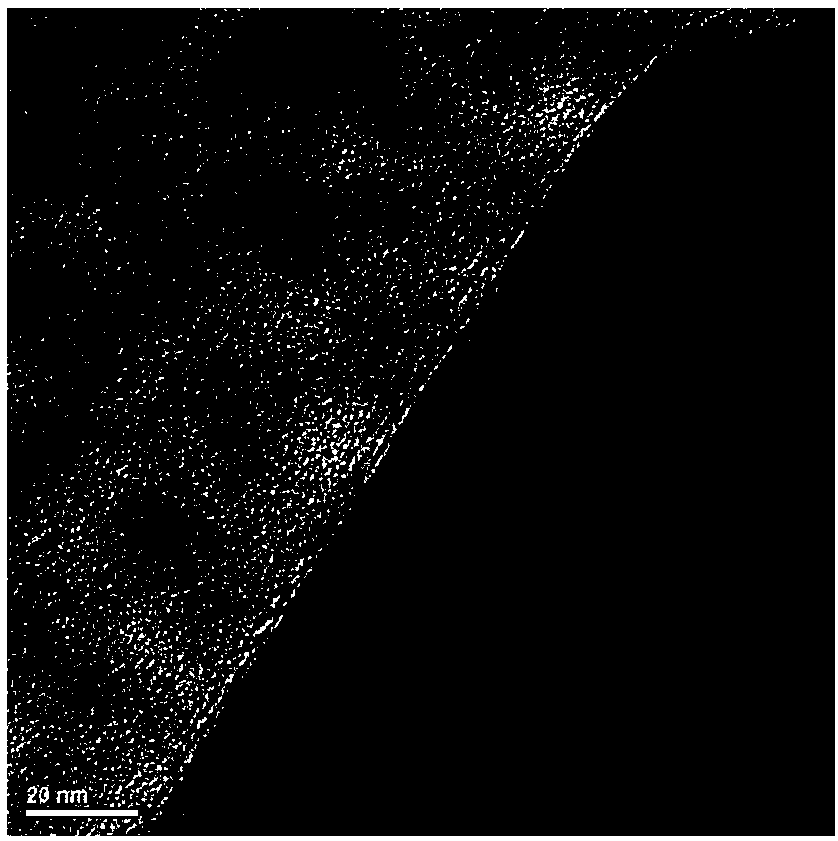
Image
Examples
Embodiment 1
[0035] 1. Add 5.67g of carbonyl iron powder into 100mL of solution and sonicate for 1h (solution 1). In order to prevent carbonyl iron from depositing in water, it is necessary to shake the beaker at intervals during the ultrasonic process to form carbonyl iron and water. homogeneous suspension;
[0036] Two, take by weighing 0.92g aluminum salt and 0.30g hydrogen phosphate di-salt and dissolve in 150mL of deionized water, mechanically stir until forming a white suspension (solution 2), wherein the mass ratio of soluble aluminum salt and hydrogen phosphate di-salt is 3:1;
[0037] 3. Pour the above solution 1 into solution 2 and stir to make it evenly mixed, then transfer the beaker containing the mixed solution to a water bath at 80°C and continue mechanical stirring until the solution evaporates to a viscous substance, and the obtained crude product Use distilled water and ethanol to carry out ultrasonic magnetic separation washing respectively, until the upper liquid is cl...
Embodiment 2
[0042] 1. Add 5.67g of carbonyl iron powder into 100mL of solution and sonicate for 1h (solution 1). In order to prevent carbonyl iron from depositing in water, it is necessary to shake the beaker at intervals during the ultrasonic process to form carbonyl iron and water. homogeneous suspension;
[0043] 2. Weigh 1.89g of aluminum salt and 0.63g of hydrogen phosphate di-salt and dissolve them in 150mL of deionized water, and mechanically stir until a white suspension (solution 2) is formed. The mass ratio of soluble aluminum salt to hydrogen phosphate di-salt 3:1;
[0044] 3. Pour the above solution 1 into solution 2 and stir to make it evenly mixed, then transfer the beaker containing the mixed solution to a water bath at 80°C and continue mechanical stirring until the solution evaporates to a viscous substance, and the obtained crude product Use distilled water and ethanol to carry out ultrasonic magnetic separation washing respectively, until the upper liquid is clear afte...
Embodiment 3
[0047]1. Add 5.67g of carbonyl iron powder into 100mL of solution and sonicate for 1h (solution 1). In order to prevent carbonyl iron from depositing in water, it is necessary to shake the beaker at intervals during the ultrasonic process to form carbonyl iron and water. homogeneous suspension;
[0048] 2. Weigh 4.26g of aluminum salt and 1.42g of hydrogen phosphate di-salt and dissolve them in 150mL of deionized water, and mechanically stir until a white suspension (solution 2) is formed. The mass ratio of soluble aluminum salt to hydrogen phosphate di-salt 3:1;
[0049] 3. Pour the above solution 1 into solution 2 and stir to make it evenly mixed, then transfer the beaker containing the mixed solution to a water bath at 80°C and continue mechanical stirring until the solution evaporates to a viscous substance, and the obtained crude product Use distilled water and ethanol to carry out ultrasonic magnetic separation washing respectively, until the upper liquid is clear after...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com