Soluble micro-needle containing slightly-soluble contraceptives and preparation method of soluble micro-needle
A contraceptive drug and insoluble technology, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, microneedles, etc., can solve the problems of slow drug transdermal rate and achieve improved transdermal penetration efficiency, Avoid stimulant effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
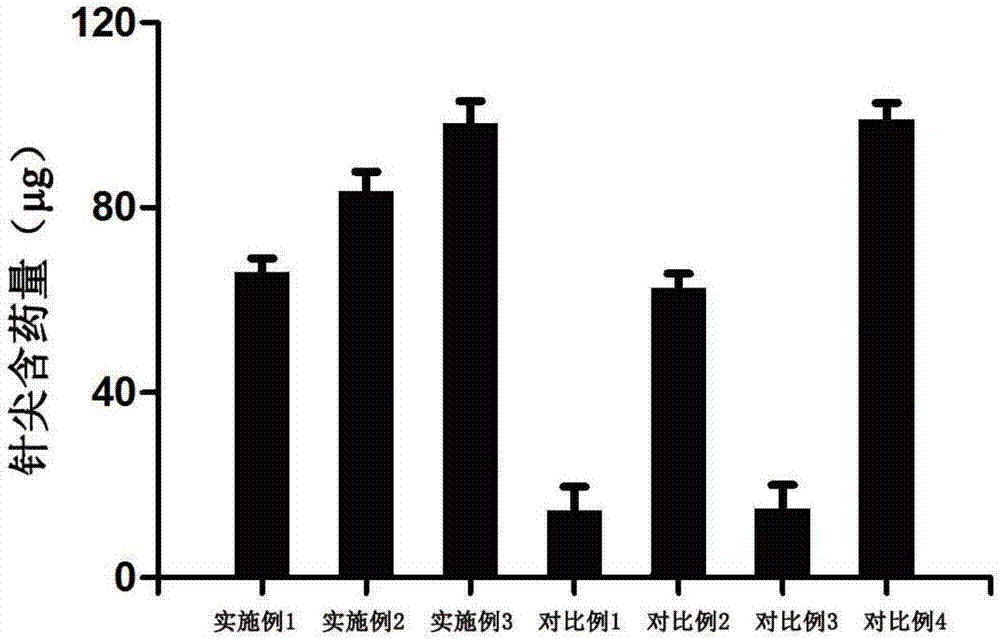
Image
Examples
Embodiment 1
[0044] This embodiment provides a soluble microneedle containing a poorly soluble contraceptive drug and a preparation method thereof, the preparation method comprising the following steps:
[0045] 1) Preparation of base solution
[0046] Weigh 1 g of dextran 40 and dissolve it in 2 mL of deionized water, and swell overnight to obtain a base solution.
[0047] 2) Preparation of levonorgestrel cyclodextrin inclusion compound
[0048] Weigh 2g hydroxypropyl-β-cyclodextrin (1.3×10^ -3 mol) was dissolved in 2mL deionized water, and after stirring until completely dissolved, 0.03g levonorgestrel (9.6×10^ -5 mol), the mol ratio of levonorgestrel to hydroxypropyl-β-cyclodextrin is 1:13, and the magnetic stirring reaction is 2h, and then the solution is freeze-dried for 48h to obtain the levonorgestrel-cyclodextrin package compound.
[0049] 3) Preparation of needle tip solution
[0050] Weigh 1 g of dextran 40, dissolve it in 2 mL of deionized water, and swell overnight to obta...
Embodiment 2
[0054] This embodiment provides a soluble microneedle containing a poorly soluble contraceptive drug and a preparation method thereof, the preparation method comprising the following steps:
[0055] 1) Preparation of base solution
[0056] With embodiment 1.
[0057] 2) Preparation of levonorgestrel cyclodextrin inclusion compound
[0058] Weigh 6g hydroxypropyl-β-cyclodextrin (3.9×10^ -3 mol) was dissolved in 6mL deionized water, and after stirring until completely dissolved, 0.03g levonorgestrel (9.6×10^ -5 mol), the mol ratio of levonorgestrel to hydroxypropyl-β-cyclodextrin is 1:40, magnetic stirring reaction 2h, then this solution is freeze-dried 48h, obtains levonorgestrel cyclodextrin package compound.
[0059] 3) Preparation of needle tip solution
[0060] With embodiment 1.
[0061] 4) Preparation of soluble microneedles loaded with levonorgestrel
[0062] With embodiment 1.
Embodiment 3
[0064] This embodiment provides a soluble microneedle containing a poorly soluble contraceptive drug and a preparation method thereof, the preparation method comprising the following steps:
[0065] 1) Preparation of base solution
[0066] With embodiment 1.
[0067] 2) Preparation of levonorgestrel cyclodextrin inclusion compound
[0068] With embodiment 1.
[0069] 3) Preparation of needle tip solution
[0070] Weigh 1 g of dextran 40, dissolve it in 2 mL of deionized water, and swell overnight to obtain a dextran solution; weigh 1 g of levonorgestrel cyclodextrin inclusion compound and dissolve it in 1 mL of deionized water to obtain a drug-containing solution. Mix the dextran solution and the drug-containing solution at a ratio of 1:2 (v / v) to obtain the needle tip solution.
[0071] 4) Preparation of soluble microneedles loaded with levonorgestrel
[0072] With embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com