Methods of bladder cancer treatment with ciclopirox, ciclopirox olamine, or a ciclopirox prodrug
A technique for ciclopirox amine and bladder cancer, which is applied in the field of treating bladder cancer with ciclopirox, ciclopirox amine or prodrug of ciclopirox
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
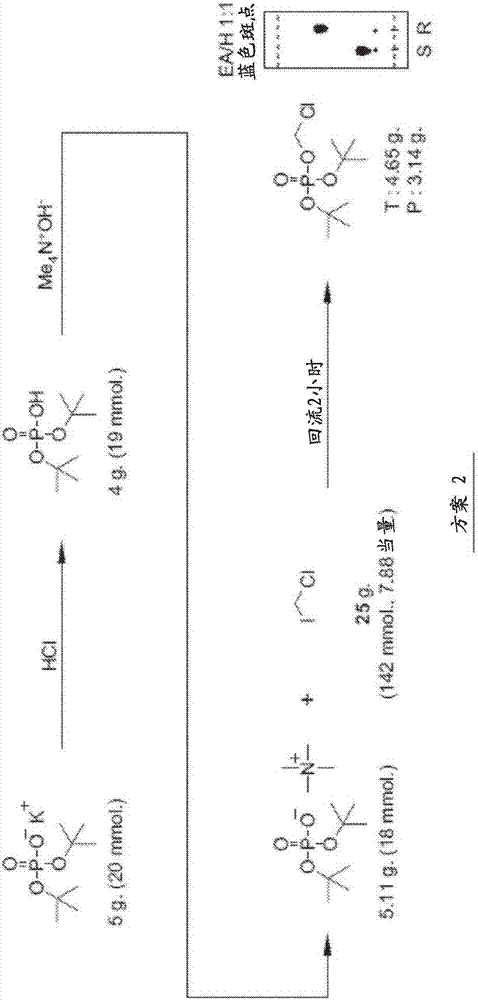
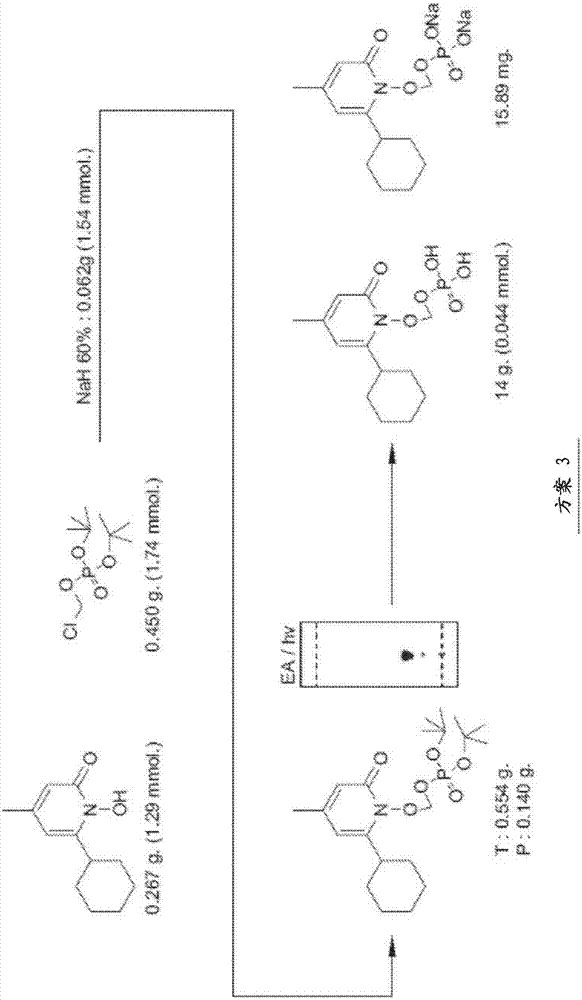
[0124] The data in the Figures and Tables demonstrate that the ciclopirox-POM prodrugs described herein are an improvement for administration to subjects. It was found that ciclopirox-POM is an improvement in chemical formulation when ciclopirox is readily bioavailable when administered IV as a prodrug compared to ciclopirox amine administered IV to mice, rats and dogs. However, after oral administration to mice, the bioavailability was low, 21% after administration of ethanolamine salt and 12% after oral administration of ciclopirox-POM prodrug. Therefore, ciclopirox is readily bioavailable when administered IV in prodrug form. Since the prodrug was undetectable in plasma and urine following IV, oral, subcutaneous, and intraperitoneal administration, it appears that when the prodrug reaches the systemic circulation, it is rapidly and completely metabolized to ciclopirox. Therefore, the ciclopirox-POM prodrug has advantages over ciclopirox amine from the viewpoint of physicoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com