Method for effectively preparing high-purity 1H-tebuconazole
A tebuconazole and high-purity technology is applied in the efficient preparation of high-purity 1H-tebuconazole, in the field of safety, and can solve the problems of inability to completely remove the purity of the solvent, difficult and unsatisfactory solvent distillation and recovery, and achieve high selectivity, Good thermal stability and the effect of reducing production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
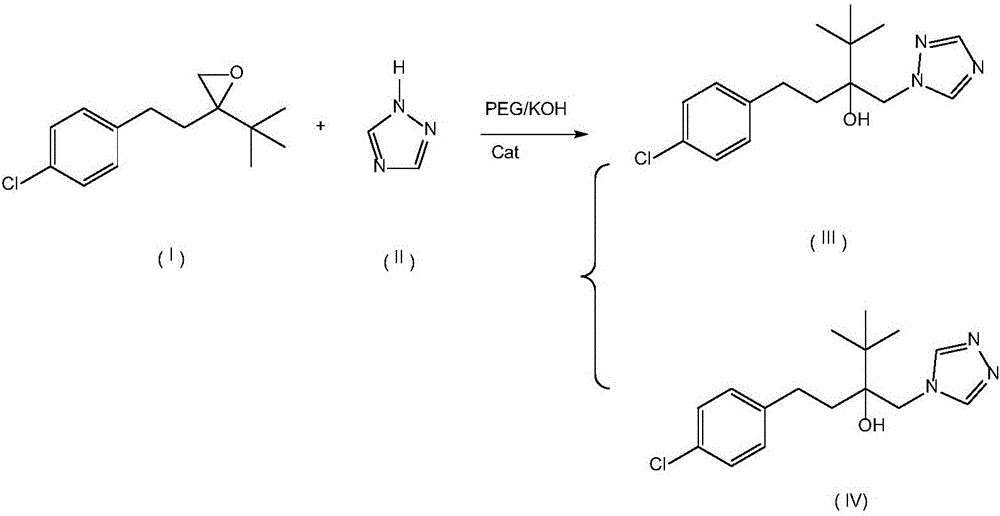
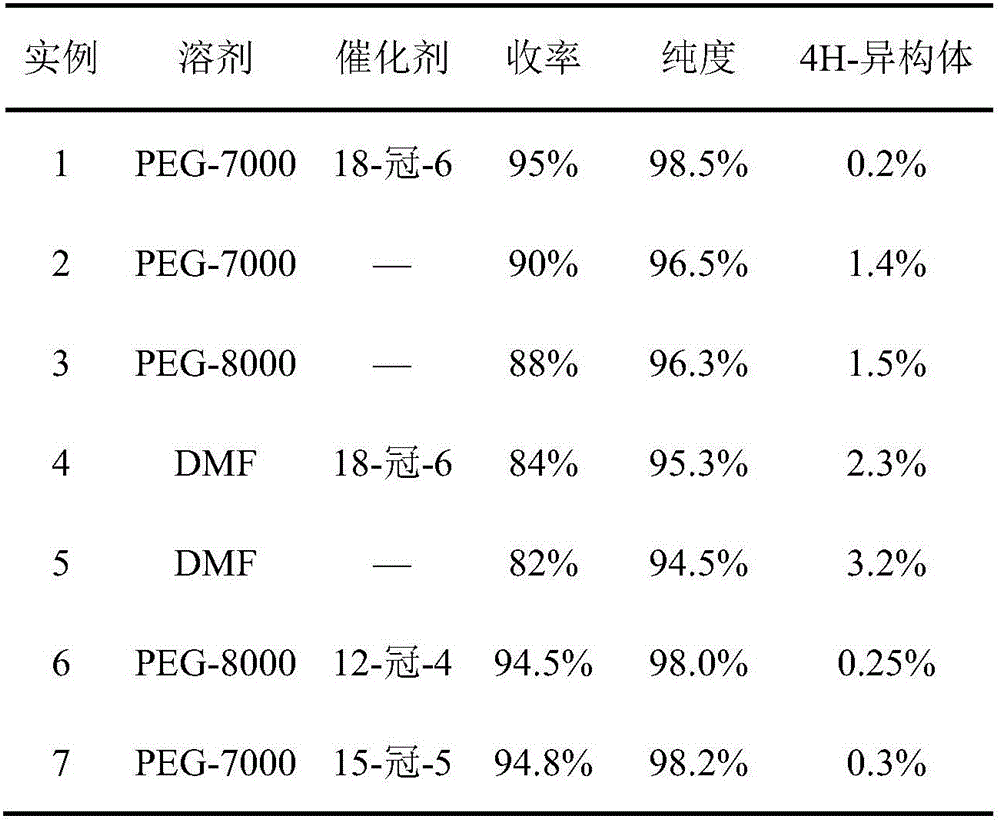
[0024] Example 1: Put 200g 2-(4-chlorophenethyl)-2-tert-butyl oxirane, 200g triazole, 90g PEG-7000, 38g KOH and 0.6g 18- Crown-6, add 400g of 2-(4-chlorophenethyl)-2-tert-butyl oxirane into the dropping funnel to be added dropwise. Start stirring, and when the temperature rises to 108°C, start to drop 2-(4-chlorophenethyl)-2-tert-butyl oxirane, control the temperature in the reaction bottle not to exceed 112°C, add dropwise for 2 hours, after the dropwise addition , control the temperature in the reaction bottle at 108-112°C, keep it warm for 5 hours; then raise the temperature to 125-128°C, keep it warm for 8 hours, take a sample test, the raw material peak is less than 0.5%, 4-H isomer 0.3%, add to the reaction bottle Sufficient water and methylcyclohexane, wherein the mass ratio of water and methylcyclohexane is 1:3.5; after layering, the water layer cools down and crystallizes to precipitate PEG, and the solid PEG is recovered by suction filtration; the organic layer cools...
Embodiment 2
[0027] Embodiment 2: drop into 200g 2-(4-chlorophenethyl)-2-tert-butyl oxirane, 180g triazole, 60g PEG-7000 and 30g KOH in the clean 1000ml four-necked bottle, mix 400g 2- (4-Chlorophenethyl)-2-tert-butyl oxirane was added to the dropping funnel to be added dropwise. Start stirring, and when the temperature rises to 105°C, start to add 2-(4-chlorophenethyl)-2-tert-butyloxirane dropwise, and control the temperature in the reaction bottle not to exceed 112°C, and add dropwise for 2 hours. After the dropwise addition, control the temperature in the reaction bottle at 108-112°C and keep it warm for 5h; then raise the temperature to 120-125°C and keep it warm for 8h. Sampling test shows that the raw material peak is less than 0.5%, and the 4-H isomer is 1.5%. Add enough water and methylcyclohexane into the reaction bottle, wherein the mass ratio of water and methylcyclohexane is 1:3; after layering, the water layer cools down and crystallizes to precipitate PEG, and the solid PEG i...
Embodiment 3
[0029] Embodiment 3: drop into 200g 2-(4-chlorophenethyl)-2-tert-butyl oxirane, 240g triazole, 120g PEG-8000 and 48g KOH in the clean 1000ml four-necked bottle, 400g 2- (4-Chlorophenethyl)-2-tert-butyl oxirane was added to the dropping funnel to be added dropwise. Start stirring, and when the temperature rises to 106°C, start to add 2-(4-chlorophenethyl)-2-tert-butyloxirane dropwise, and control the temperature in the reaction bottle not to exceed 112°C, and add dropwise for 6 hours. After the dropwise addition, control the temperature in the reaction bottle at 108-112°C and keep it warm for 5 hours; then raise the temperature to 128-130°C and keep it warm for 8 hours; sampling test shows that the raw material peak is less than 0.5%, and the 4-H isomer is less than 1.7%. Add enough water and methylcyclohexane into the reaction bottle, wherein the mass ratio of water and methylcyclohexane is 1:4; after layering, the water layer cools down and crystallizes to precipitate PEG, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com