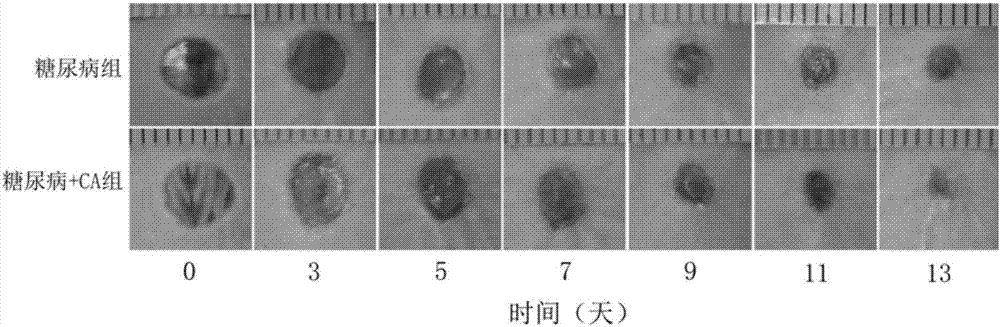
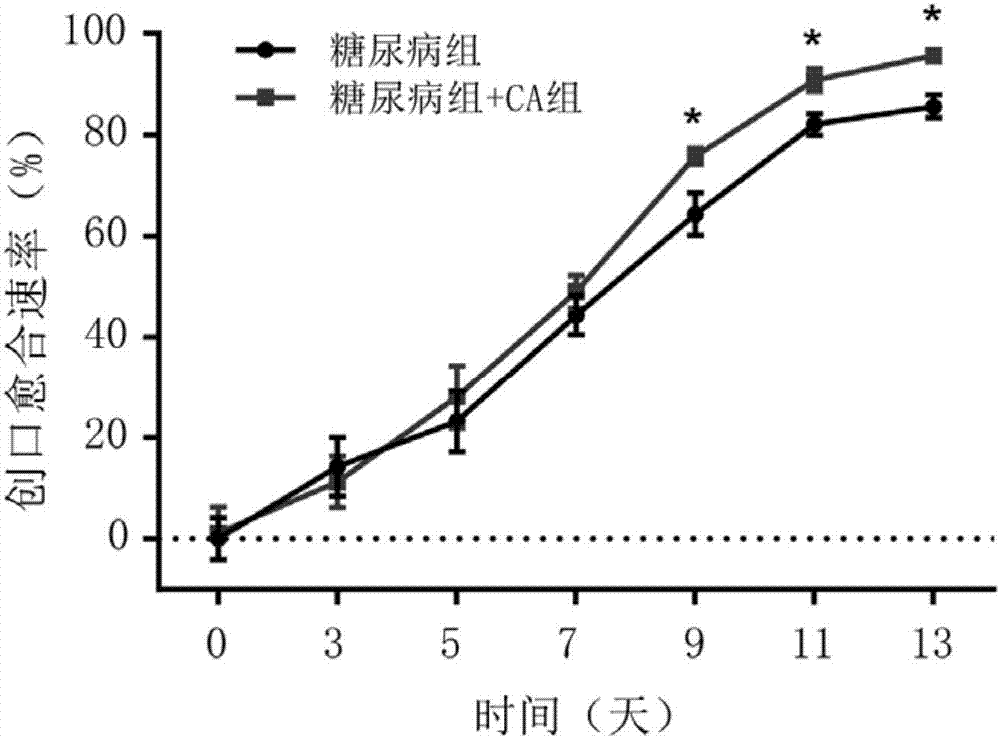
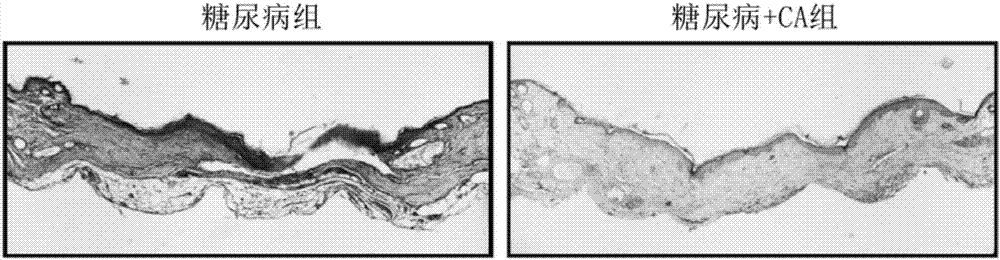
Medicine for treating diabetic ulcer
A technology for diabetic ulcers and drugs, applied in the field of biomedicine, can solve the problems of Nrf2 agonist-CA application strategy is not safe and simple, and achieve good therapeutic effect, improve wound healing, and safe and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present invention will be further described below in conjunction with the accompanying drawings and embodiments.
[0014] The drug for treating diabetic ulcers in this embodiment is a gel composed of solute: cinnamaldehyde and solvent: PEG 400, and the concentration range of cinnamaldehyde in the gel is 1.2-20 μmol / L.
[0015] Taking the preparation of 1 mL of 4 μmol / L cinnamaldehyde gel as an example, the preparation method of the drug for treating diabetic ulcers in this embodiment is introduced, and the specific steps are as follows:
[0016] Step 1: Prepare 1 mL of 20 mmol / L cinnamaldehyde gel
[0017] 1) The number of moles of CA contained in 1mL 20mmol / L cinnamaldehyde gel:
[0018] 2) n=c×v
[0019] no CA =(20mmol / L)×(1mL)=20×10 -6 mol
[0020] 3) m=n×M
[0021] m CA =(20×10 -6 mol)×132.16=2.6432×10 -3 g
[0022] 4) v=m / ρ
[0023] v CA =(2.6432×10 -3 g)÷(1.05g / mL)=2.517ul
[0024] Add 2.517ul of CA into 1ml of PEG400, and shake at high speed to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com