Substituted aminopurine compounds, compositions thereof, and methods of treatment therewith
A kind of compound, the technology of the substituent, applied in the substituted aminopurine compound, its composition and its treatment field, can solve the problem such as low response rate median survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
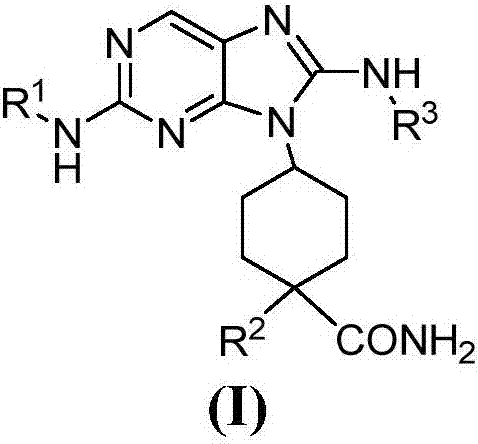
[0301] Example 1. (1s,4s)-4-(8-((4-chloro-2,6-difluorophenyl)amino)-2-((tetrahydro-2H-pyran-4-yl)amino )-9H-purin-9-yl)cyclohexane-1-carboxamide
[0302]
[0303] tert-butyl cis-(4-carbamoyl-cyclohexyl)-carbamate. cis-4-tert-butoxycarbonylamino-cyclohexanecarboxylic acid (1 equiv) and TEA (1.1 equiv) were dissolved in 0.3M THF, and the mixture was cooled to 0°C. Ethyl chloroformate (1.1 equiv) was added dropwise. After stirring at 0°C for 30 min, NH3 in THF was added. The mixture was stirred at -78°C for 2 hours. The mixture was diluted with water, and the solvent was evaporated until only water remained. The resulting precipitate was collected by filtration and dried under vacuum to give cis-(4-carbamoyl-cyclohexyl)-carbamate tert-butyl ester (45%) as a white solid. 1 H NMR (400MHz, DMSO-d 6)δppm 7.10(brs,1H),6.69(brs,2H),3.41(brs,1H),2.10(m,1H),1.72(m,2H),1.53(m,2H),1.42(m,4H) ,1.36(s,9H).
[0304] cis-4-amino-cyclohexanecarboxylic acid amide hydrochloride. To a ...
Embodiment 2
[0311] Example 2. (1s,4s)-4-(2-((tetrahydro-2H-pyran-4-yl)amino)-8-((2,4,6-trichlorophenyl)amino)- 9H-purin-9-yl)cyclohexane-1-carboxamide
[0312]
[0313] (1s,4s)-4-(2-((tetrahydro-2H-pyran-4-yl)amino)-8-((2,4,6-trichlorophenyl)amino)-9H-purine- 9-yl) cyclohexane-1-carboxamide. (1s,4s)-4-((5-amino-2-((tetrahydro-2H-pyran-4-yl)amino)pyrimidin-4-yl)amino)cyclohexanecarboxamide was stirred at room temperature (1 equiv) and 1,3,5-trichloro-2-isothiocyanatobenzene (1 equiv) for 2 hours. EDC (2 equiv) in THF (0.17M) was added and the reaction was heated to 60°C and stirred for 1 hour. The reaction was cooled, and the organic solvent was concentrated well. The resulting residue was diluted with water, stirred for 30 minutes, and filtered. Standard workup and purification methods afforded (1s,4s)-4-(2-((tetrahydro-2H-pyran-4-yl)amino)-8-((2,4,6-trichlorophenyl )amino)-9H-purin-9-yl)cyclohexanecarboxamide (79% yield). 1 H NMR (400MHz, DMSO-d 6 )δppm: 8.11 (br.s., 1H) 7.69 ...
Embodiment 3
[0314] Example 3. (1s,4s)-4-(8-((2-chloro-4,5-difluorophenyl)amino)-2-((tetrahydro-2H-pyran-4-yl)amino )-9H-purin-9-yl)cyclohexanecarboxamide
[0315]
[0316] 1-Chloro-4,5-difluoro-2-isothiocyanatobenzene. To a mixture of 2-chloro-4,5-difluoroaniline (1 equiv) and sodium hydroxide (3 equiv) in DCM (0.24M) and water (0.24M) was added thiophosgene dropwise at 0 °C (3 equivalents). The reaction mixture was stirred overnight at 25°C. TLC showed the reaction was complete. The organic phase was separated and washed with MgSO 4 Drying followed by concentration afforded 1-chloro-4,5-difluoro-2-isothiocyanatobenzene (53%) as a white solid.
[0317](1s,4s)-4-(8-((2-chloro-4,5-difluorophenyl)amino)-2-((tetrahydro-2H-pyran-4-yl)amino)-9H- Purin-9-yl) cyclohexanecarboxamide. To (1s,4s)-4-((5-amino-2-((tetrahydro-2H-pyran-4-yl)amino)pyrimidin-4-yl)amino)cyclohexanecarboxamide (1 equivalent) 1-Chloro-4,5-difluoro-2-isothiocyanatobenzene (1.1 equiv) was added in one portion to a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com