A kind of method for preparing odancate intermediate
A technology of time interval and reaction system, which is applied in the direction of carboxylic acid nitrile preparation, sulfonate ester preparation, chemical instruments and methods, etc., can solve the problems of large-scale production, increased difficulty of product purification, and chiral isomer content Low-level problems, achieving significant social and economic benefits, easy separation and purification, and less solvent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
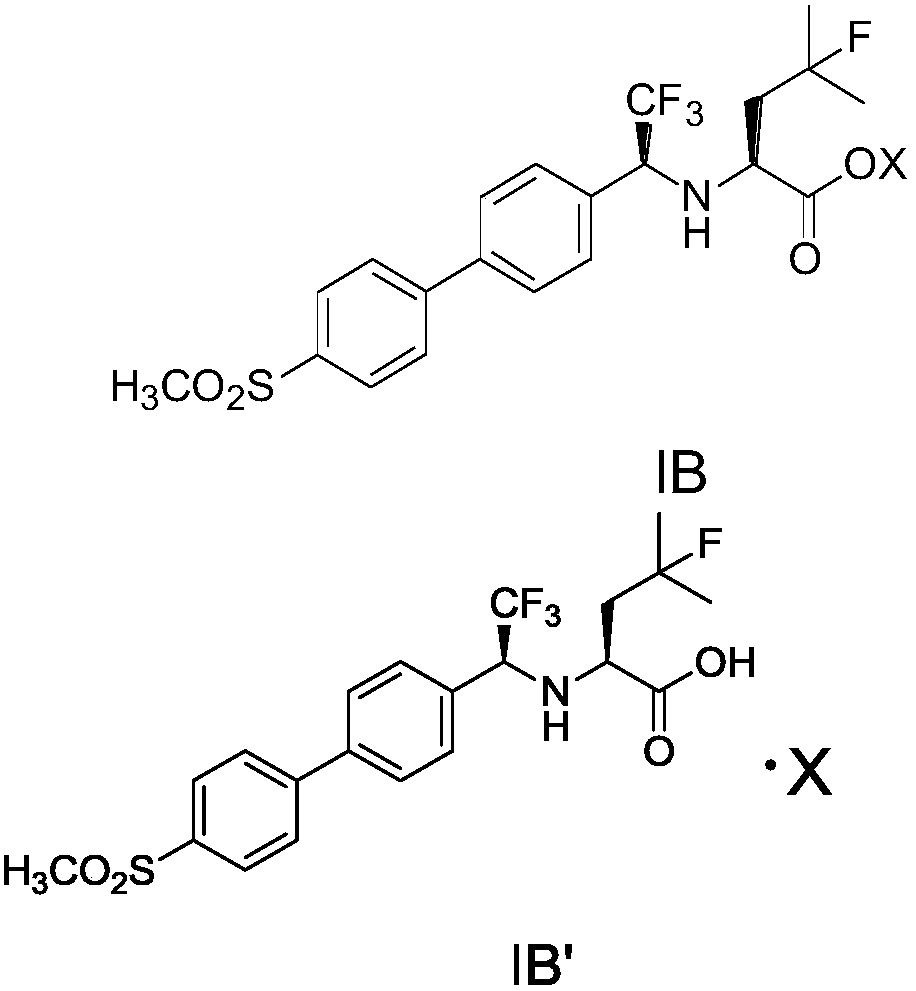
[0059] Embodiment 1: Odangcate intermediate (IA or IB')
[0060]
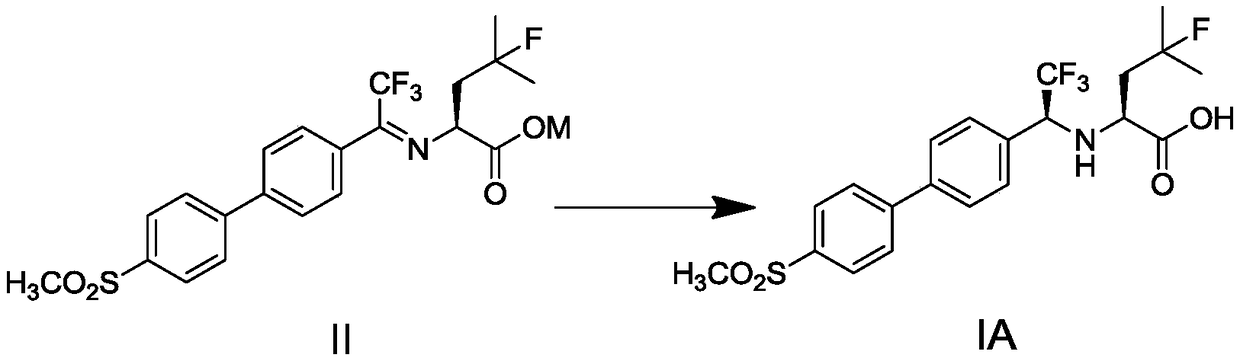
[0061] Step 1), preparation of imine carboxylate intermediate (II)
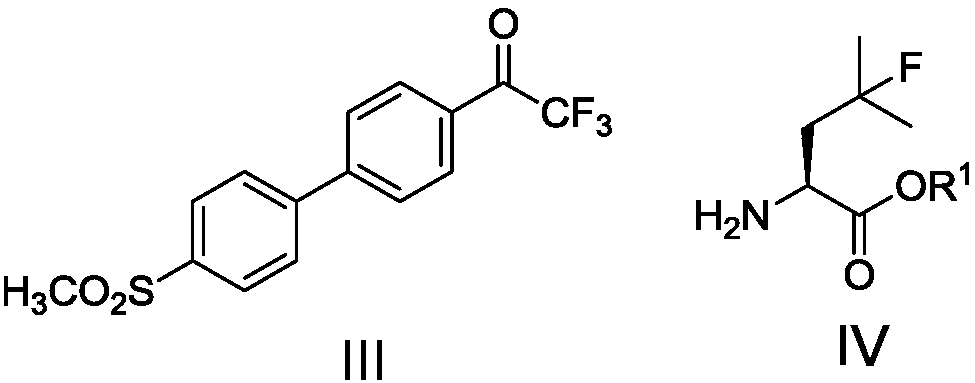
[0062] 2,2,2-trifluoro-1-(4'-(methylsulfonyl)biphenyl-4-yl)ethanone (III) 9.1g (26.2mmol, 1eq), 4-fluoro-L-leu Add 4.9g (27.5mmol, 1.05eq) of ethyl acetate (IV) into methanol, stir to dissolve, and add 9.0g (65.2mmol, 2.5eq) of anhydrous potassium carbonate. Heat the reaction system to 50±5°C for 4-5 hours. Cool to 25 ~ 30 ° C, filter off insoluble matter. The filtrate was concentrated, and 100 mL of ethyl acetate was added to the residue to make slurry for 1 hour. After filtering, the filter cake was washed with 50 mL of ethyl acetate, and dried to obtain 13.7 g of an imine carboxylate intermediate (yellow solid).
[0063] Step 2), preparation of ordancate intermediate carboxylic acid (IA)
[0064] Experimental condition A
[0065] Add 2.0g (4mmol, 1eq) of imine carboxylate intermediate (II) into 20mL of methanol, add 1.1g (8mmol, 2e...
Embodiment 2
[0077] Embodiment 2: Preparation of Odangcate (V)
[0078]
[0079] Step 1), preparation of imine carboxylate intermediate (II)
[0080] 2,2,2-Trifluoro-1-(4'-(methylsulfonyl)biphenyl-4-yl)ethanone (III) 41.8g (128mmol, 1eq), 4-fluoro-L-leucine Add 23.7g (134mmol, 1.05eq) of ethyl acetate (IV) into 200mL of methanol, stir to dissolve, and add 44.0g (319mmol, 2.5eq) of anhydrous potassium carbonate. Heat the reaction system to 50±5°C for 4-5 hours. Cool to 25 ~ 30 ° C, filter off insoluble matter. The filtrate was concentrated, and 1000 mL of ethyl acetate was added to the residue to make slurry for 1 hour. After filtering, the filter cake was washed with 200 mL of ethyl acetate, and dried to obtain 65.0 g of an imine carboxylate intermediate (yellow solid).
[0081] Step 2), preparation of ordancate intermediate carboxylic acid (IA)
[0082] Add 65.0g (128mmol, 1eq) of imine carboxylate intermediate (II) to 300mL methanol, add 35.0g (256mmol, 2eq) of anhydrous zinc chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com