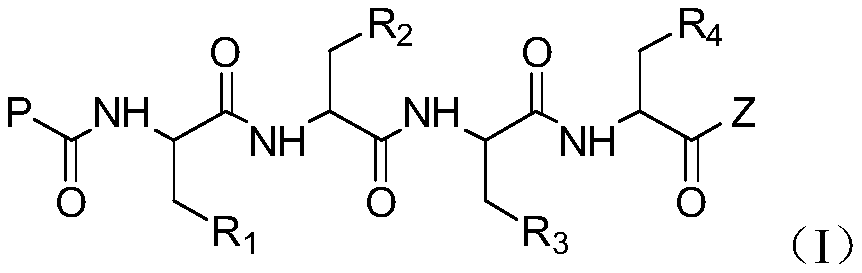
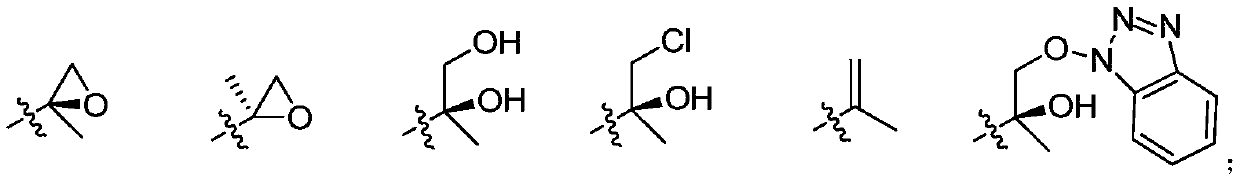
A kind of tetrapeptide propylene oxide derivative and its preparation method and use
A technology of peptide propylene oxide and propylene oxide, which is applied to novel tetrapeptide propylene oxide derivatives and their application fields in pharmacodynamics, and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0117] Synthesis of the first part of the compound
[0118] The preparation of the compound of the present invention can be carried out according to the following process:
[0119] One, the preparation of compound (I)
[0120]
[0121] 1. Preparation of compound (I-3):
[0122] Dissolve compound (I-1), HOBt in anhydrous DCM and stir at -5°C for 10 min, then add EDIHCl at this temperature, stir for 15-20 min, then add compound (I-2) and stir for 15-20 min, then add DIPEA was stirred for 20 minutes, then moved to room temperature for reaction. After the reaction was complete, it was poured into water, extracted with DCM, and the combined organic phases were washed with dilute HCl, sodium bicarbonate solution, and saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain compound (I-3).
[0123] 2. Preparation of compound (I-4):
[0124] Compound (I-3) was dissolved in anhydrous DCM, TFA was slowly added dropwise at -5°C, stirred for 0.5 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com