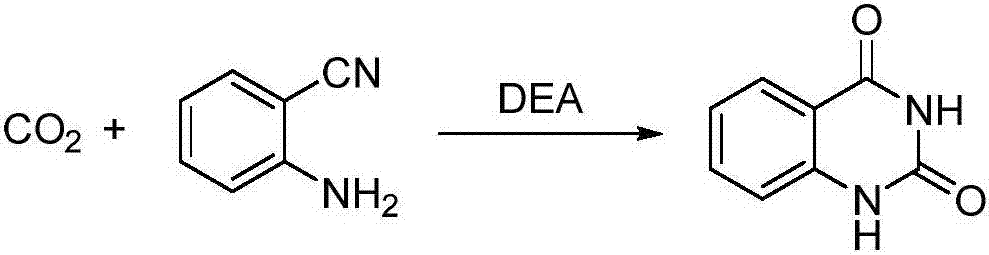
Synthetic method of quinazoline-2,4(1H, 3H)-dione and derivatives thereof
A synthesis method and quinazoline technology are applied in the field of synthesis of quinazoline-2,4-dione and derivatives thereof, which can solve the problems of industrial application limitation of reaction conditions, harsh reaction conditions, complicated processes, etc. The method is simple and controllable, with low cost and good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Weigh 0.59g (5mmol) of o-aminobenzonitrile and place it in the polytetrafluoroethylene lining of a stainless steel reaction kettle, add 3mL of an aqueous solution of diethanolamine with a concentration of 1mol / L, stir for 2 minutes and mix well, then introduce carbon dioxide and heat up To 100° C., adjust the carbon dioxide pressure to 1 MPa to carry out the carboxycyclization reaction under stable conditions, and the reaction time is 12 hours. After the reaction, cool the reaction system to room temperature, slowly release unreacted carbon dioxide, add 10 mL of deionized water and stir to disperse the product, filter the precipitate and wash it with a small amount of distilled water, then wash it three times with 15 mL of methyl tert-butyl ether each time, After drying at 100°C, the product was quinazoline-2,4(1H,3H)-dione with a yield of 94%.
Embodiment 2
[0021] Weigh 0.68g (5mmol) of 2-amino-5-fluorobenzonitrile and place it in the polytetrafluoroethylene lining of a stainless steel reaction kettle, add 3mL of a diethanolamine aqueous solution with a concentration of 0.33mol / L, stir for 2 minutes and mix well , feed carbon dioxide and raise the temperature to 100° C., adjust the pressure of carbon dioxide to 1 MPa to carry out carboxycyclization reaction under stable conditions, and the reaction time is 12 hours. After the reaction, cool the reaction system to room temperature, slowly release unreacted carbon dioxide, add 10 mL of deionized water and stir to disperse the product, filter the precipitate and wash it with a small amount of distilled water, then wash it three times with 15 mL of methyl tert-butyl ether each time, The product was dried at 100° C. to obtain 6-fluoroquinazoline-2,4(1H,3H)-dione with a yield of 94%.
Embodiment 3
[0023] Weigh 0.763g (5mmol) of 2-amino-5-chlorobenzonitrile and place it in the polytetrafluoroethylene lining of the stainless steel reaction kettle, add 3mL of diethanolamine aqueous solution with a concentration of 1.33mol / L, stir for 2 minutes and mix well , feed carbon dioxide and raise the temperature to 100° C., adjust the pressure of carbon dioxide to 1 MPa to carry out carboxycyclization reaction under stable conditions, and the reaction time is 12 hours. After the reaction, cool the reaction system to room temperature, slowly release unreacted carbon dioxide, add 10 mL of deionized water and stir to disperse the product, filter the precipitate and wash it with a small amount of distilled water, then wash it three times with 15 mL of methyl tert-butyl ether each time, The product 6-chloroquinazoline-2,4(1H,3H)-dione was dried at 100° C. with a yield of 93%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com