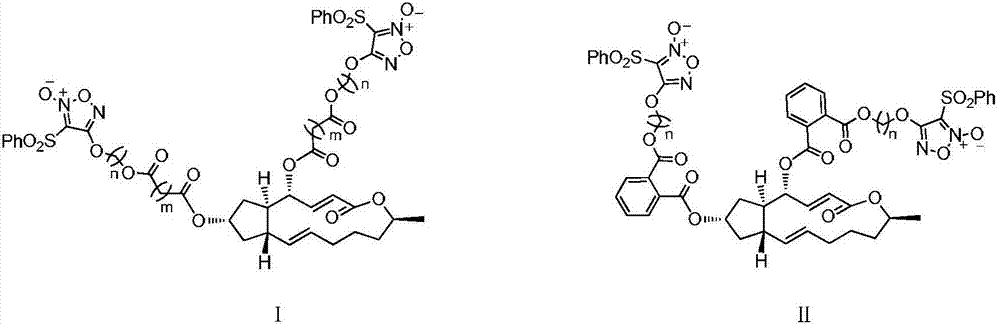
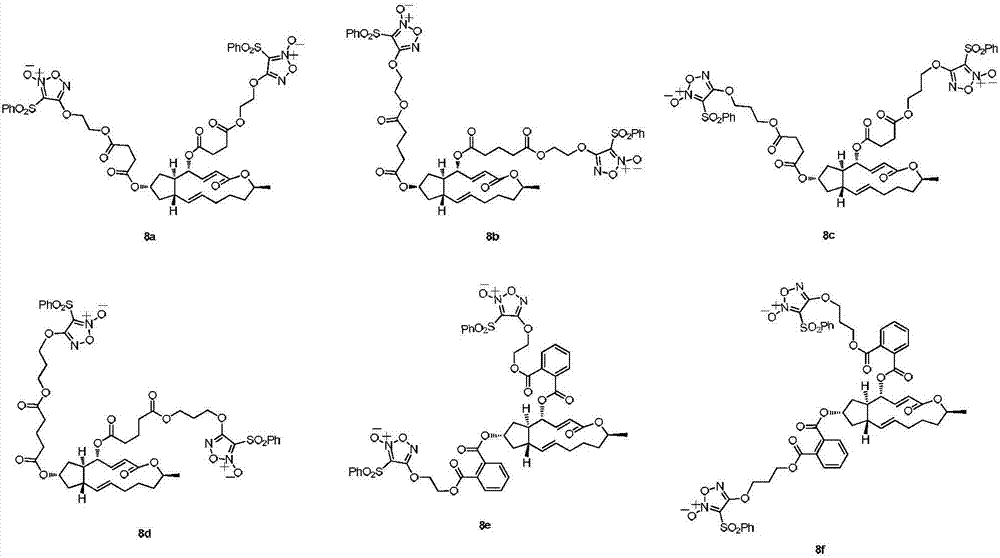
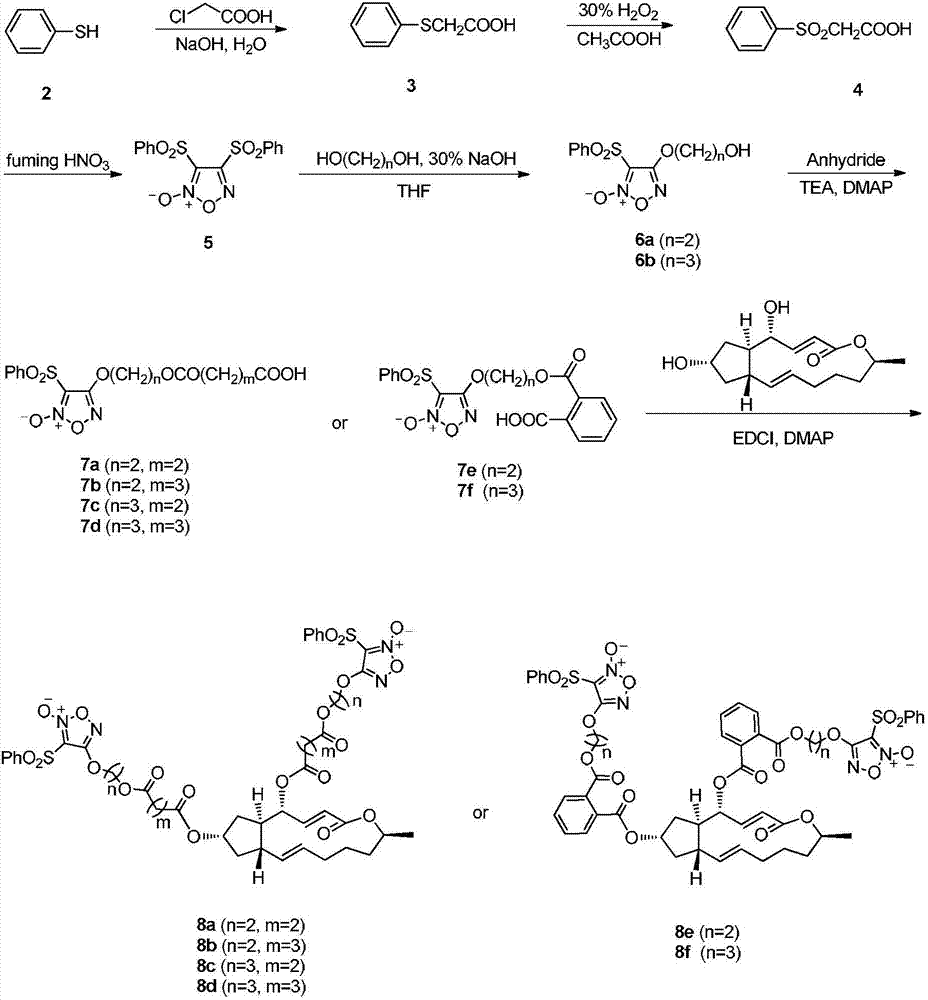
4,7-site difurazan NO donor substituted derivatives of brefeldin A, and preparation method and uses thereof
A technology of brefeldin and feldspar, which is applied to the derivatives modified at the 4 and 7 positions of brefeldin A, to prepare anti-tumor drugs, which can solve the problems of poor bioavailability, low water solubility, Issues such as neurotoxicity limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] 60g NaOH and 480mL H 2 The solution prepared by O was poured into a reaction flask, thiophenol (75mL, 0.63mol) was taken, and then chloroacetic acid (78g, 0.825mol) was added, and a large amount of white precipitates were precipitated in the reaction solution. 6N HCl was added to obtain phenylthioacetic acid (3) as a white solid. Dissolve 3 (20g, 0.12mol) in 90mL glacial acetic acid, add 24.3mL 30% H 2 o 2 , stirring at room temperature. After the reaction is complete, add fuming HNO 3 48mL. Heating reaction, after 4 hours, white needle-like crystals precipitated, filtered and dried to obtain 3,4-diphenylsulfonylfurazan nitrogen oxide (5). Ethylene glycol (0.56mL, 10mmol) and 5 (1g, 2.7mmol) were dissolved in 10mL THF, and 30% NaOH aqueous solution (0.5mL, 3mmol) was added dropwise to react for 2h. Pour into 20mL H 2 O, extracted with EtOAc (3×20 mL), the organic layers were combined, washed once with saturated brine, dried over anhydrous sodium sulf...
Embodiment 2
[0036]
[0037] Referring to the synthetic method of Example 1. Yellow oil, yield 13.4%; 1 H-NMR (400MHz, DMSO-d 6 )δ7.72~8.02(10H,m,Ar-H),7.23(1H,dd,J=15.70Hz and 3.29Hz,C 3 -H),5.73(1H,m,C 11 -H),5.60(1H,dd,J=15.80Hz and 1.69Hz,C 2 -H),5.28(1H,m,C 10 -H),5.16(1H,dd,J=15.14Hz and 9.75Hz,C 4 -H),5.01(1H,m,C 7 -H),4.73(1H,m,C 15 -H),4.43~4.62(8H,m,2OC H 2 C H 2 O),2.39~2.63(8H,m,2COC H 2 CH 2 C H 2 CO),0.73~2.34(19H,m,C 5 ,2C 6 ,2C 8 ,C 9 ,2C 12 ,2C 13 ,2C 14 -H,2COCH 2 C H 2 CH 2 CO,C H 3 ); 13 C-NMR (400MHz, DMSO-d 6 )δ172.83,172.29,172.05,171.56,164.86,158.69,158.69,148.34,137.21,136.13,136.13,136.10,135.78,130.68,129.97,129.97,129.97,129.97,128.31,128.31,128.31,128.31,117.13,110.46,110.46 ,75.76,74.74,71.26,69.33,69.33,61.28,61.24,51.28,42.43,42.40,37.43,33.40,32.71,32.66,32.40,32.28,31.27,26.26,20.57,19.82,19.7 HR-ES ( +Na)m / z: calcd for C 46 h 52 N 4 o 20 S 2 :1067.2509, found 1067.2510.
Embodiment 3
[0039]
[0040] Referring to the synthetic method of Example 1. Yellow oil, yield 8.7%; 1 H-NMR (400MHz, DMSO-d 6 )δ7.71~8.01(10H,m,Ar-H),7.22(1H,dd,J=15.86Hz and 3.25Hz,C 3 -H),5.74(1H,m,C 11 -H),5.63(1H,dd,J=15.80Hz and 1.67Hz,C 2 -H),5.25(1H,m,C 10 -H),5.18(1H,dd,J=15.04Hzand 9.74Hz,C 4 -H),4.94(1H,m,C 7 -H),4.71(1H,m,C 15 -H),4.15~4.45(8H,t,J=6.13Hz,2OC H 2 CH 2 C H 2 O),2.52~2.70(8H,m,2COC H 2 C H 2 CO),0.72~2.52(19H,m,C 5 ,2C 6 ,2C 8 ,C 9 ,2C 12 ,2C 13 ,2C 14 -H,2OCH 2 C H 2 CH 2 O,C H 3 ); 13 C-NMR (400MHz, DMSO-d 6 )δ172.36,172.36,171.83,171.40,165.26,159.15,159.15,148.45,137.53,137.50,136.50,136.50,136.16,131.03,130.37,130.37,130.37,130.37,128.72,128.72,128.72,128.72,117.60,110.88,110.88 HR-ES +Na)m / z: calcd for C 46 h 52 N 4 o 20 S 2 :1067.2509, found 1067.2456.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com