Brefeldin A derivatives, and preparation method and uses thereof
A Brefeldin and Fideloid technology, which can be used in drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as poor bioavailability, neurotoxicity limitation, and low water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
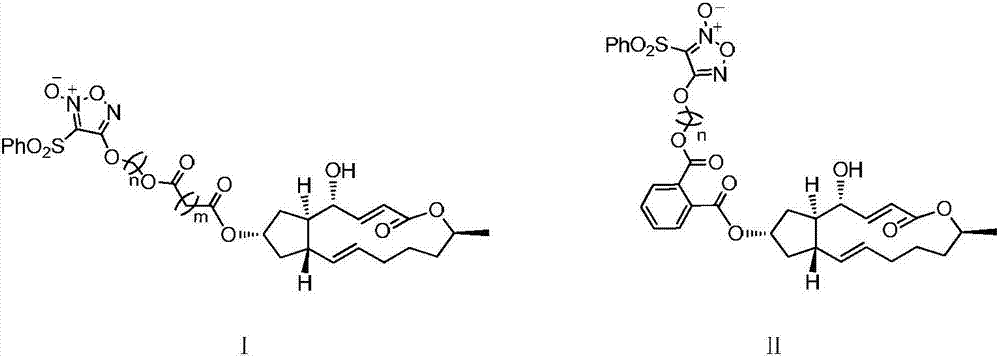
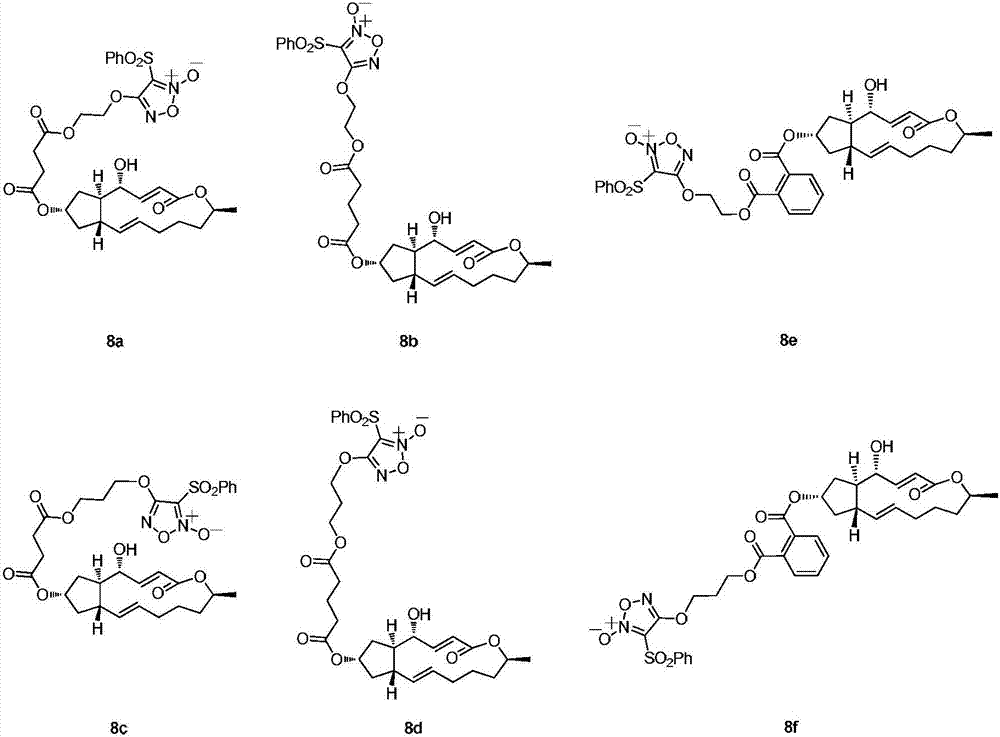
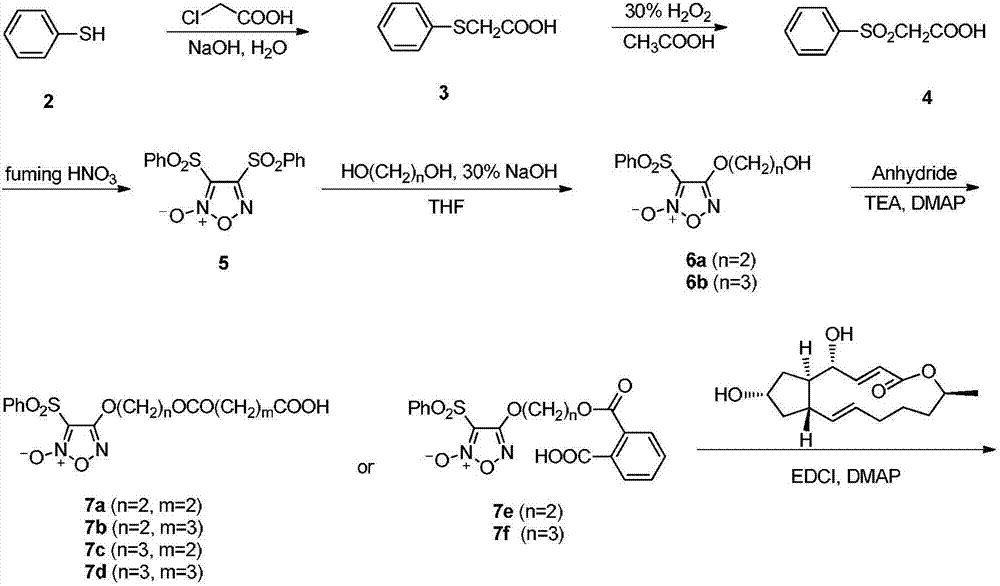
Image
Examples
Embodiment 1
[0039]
[0040] 60g NaOH and 480mL H 2 The solution prepared by O was poured into a reaction flask, thiophenol (75mL, 0.63mol) was taken, and then chloroacetic acid (78g, 0.825mol) was added, and a large amount of white precipitates were precipitated in the reaction solution. 6N HCl was added to obtain phenylthioacetic acid (3) as a white solid. Dissolve 3 (20g, 0.12mol) in 90mL glacial acetic acid, add 24.3mL 30% H 2 o 2 , stirring at room temperature. After the reaction is complete, add fuming HNO 3 48mL. Heating reaction, after 4 hours, white needle-like crystals precipitated, filtered and dried to obtain 3,4-diphenylsulfonylfurazan nitrogen oxide (5). Ethylene glycol (0.56mL, 10mmol) and 5 (1g, 2.7mmol) were dissolved in 10mL THF, and 30% NaOH aqueous solution (0.5mL, 3mmol) was added dropwise to react for 2h. Pour into 20mL H 2 O, extracted with EtOAc (3×20 mL), the organic layers were combined, washed once with saturated brine, dried over anhydrous sodium sulf...
Embodiment 2
[0042]
[0043] Referring to the synthetic method of Example 1. Yellow oil, yield 8.1%; 1 H-NMR (400MHz, DMSO-d 6 )δ7.73~7.99(5H,m,Ar-H),7.32(1H,dd,J=15.61Hz and 2.96Hz,C 3 -H),5.70(2H,m,C 2 ,C 11 -H),5.19(1H,d,J=5.62Hz,O H ),5.14(1H,dd,J=15.12Hz and 9.64Hz,C 10 -H),5.00(1H,m,C 7 -H),4.70(1H,m,C 15 -H),4.39~4.60(4H,t,J=4.24Hz,OC H 2 C H 2 O),4.00(1H,m,C 4 -H),2.32~2.37(4H,m,COC H 2 CH 2 C H 2 CO),0.73~2.46(17H,m,C 5 ,2C 6 ,2C 8 ,C 9 ,2C 12 ,2C 13 ,2C 14 -H,COCH 2 C H 2 CH 2 CO,C H 3 ); 13 C-NMR (400MHz, DMSO-d 6 )δ172.30,172.09,165.60,158.69,154.03,137.20,136.27,136.14,130.12,129.98,129.98,128.31,128.31,116.47,110.46,74.99,74.00,70.89,69.33,61.25,51.88,42.69,38.16,38.07,33.43 , 32.71, 32.38, 31.37, 26.38, 20.69, 19.79; HR-MS (ESI, M+Na) m / z: calcd for C 31 h 38 N 2 o 12 S: 685.2038, found 685.2039.
Embodiment 3
[0045]
[0046] Referring to the synthetic method of Example 1. Yellow oil, yield 8.6%; 1 H-NMR (400MHz, CDCl 3 )δ7.63~8.10(5H,m,Ar-H),7.34(1H,dd,J=15.69Hz and 3.09Hz,C 3 -H),5.91(1H,dd,J=15.63Hzand 1.89Hz,C 2 -H),5.71(1H,m,C 11 -H),5.21(1H,dd,J=15.22Hz and 9.65Hz,C 10 -H),5.08(1H,m,C 7 -H),4.86(1H,m,C 15 -H),4.30~4.51(4H,t,J=6.17Hz,OC H 2 CH 2 C H 2 O),4.12(1H,m,C 4 -H),2.59~2.64(4H,m,COC H 2 C H 2 CO),0.71~2.38(17H,m,C 5 ,2C 6 ,2C 8 ,C 9 ,2C 12 ,2C 13 ,2C 14 -H,OCH 2 C H 2 CH 2 O,C H 3 ); 13 C-NMR (400MHz, CDCl 3 )δ172.37,171.88,165.98,159.16,154.42,137.51,136.68,136.53,130.46,130.38,138.38,128.73,128.73,116.83,110.91,75.64,74.37,71.27,68.55,60.71,52.28,43.05,38.56,38.45,33.81 ,31.77,30.17,29.25,29.00,27.75,21.07; HR-MS(ESI,M+Na) m / z: calcd for C 31 h 38 N 2 o 12 S: 685.2038, found 685.2035.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com