Dry powder inhaler
A dry powder inhaler and carrier plate technology, used in inhalers, drug devices, other medical devices, etc., can solve the problems of influence of clean conditions, poor sealing, easy to cause pollution, etc., to reduce production and management costs, prevent Button offset, easy cleaning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
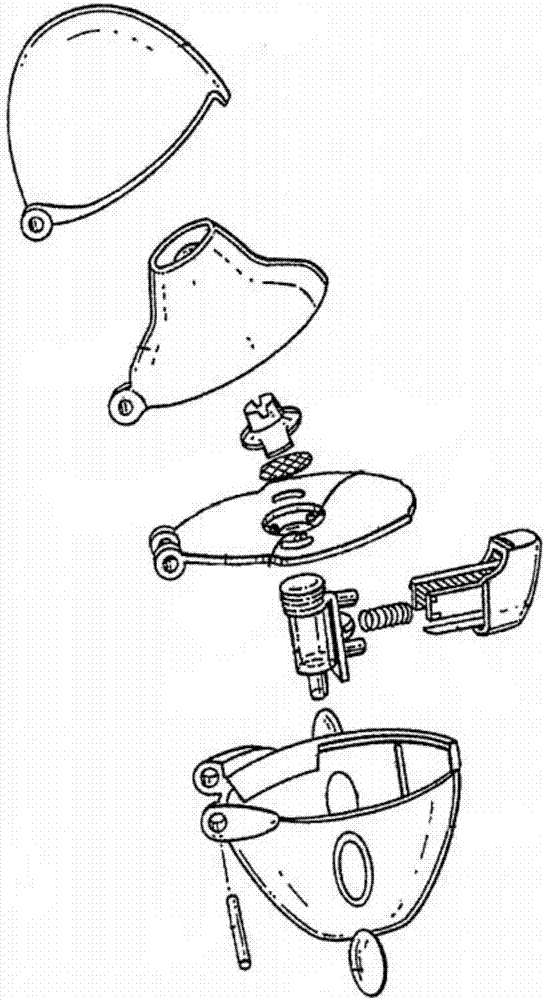
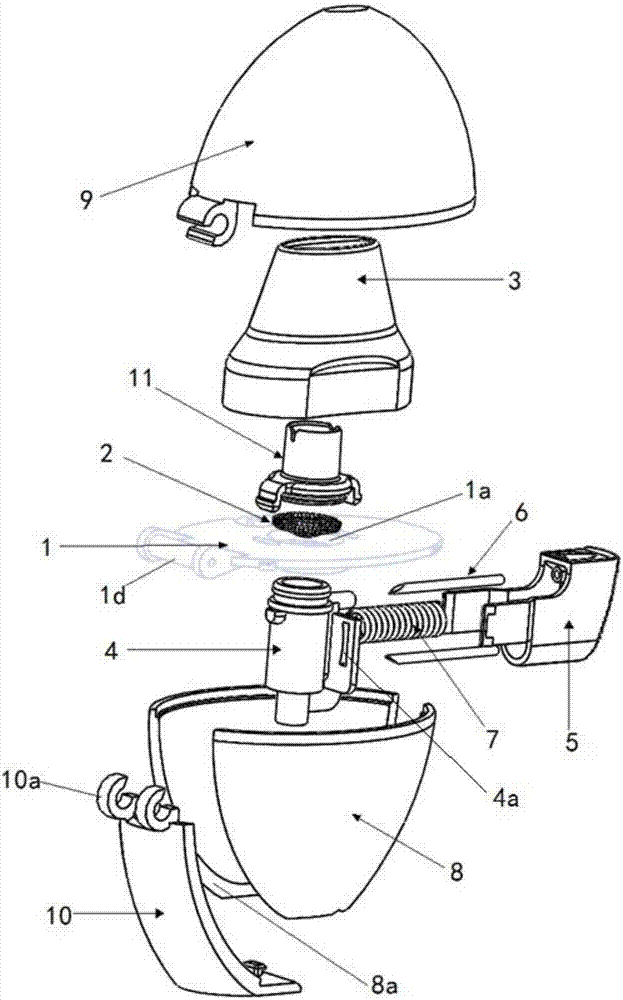
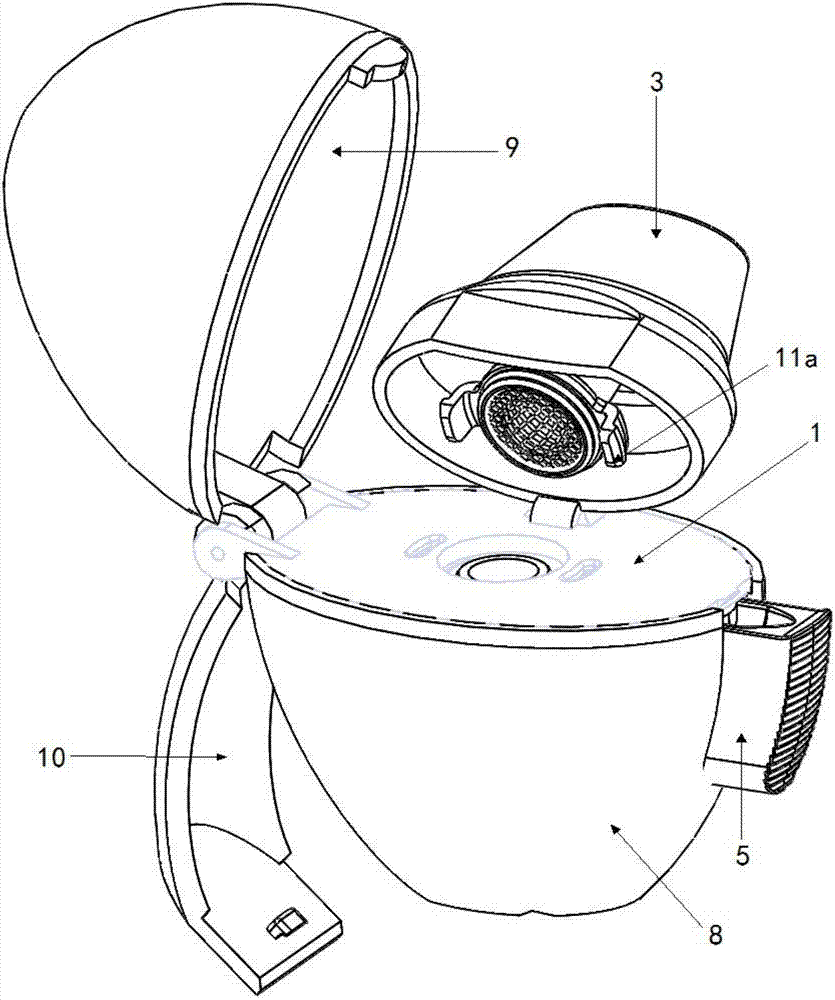
[0086] Figure 2 to Figure 7 A preferred embodiment of the dry powder inhaler of the present application is shown, which is the same as the traditional structure, and it also includes: a loading plate 1, a screen 2, a suction nozzle 3, a capsule chamber 4, a button 5, a spring 7, a lower Structural parts such as shell 8 and upper shell 9. in:
[0087] A through hole 1 a is formed on the supporting plate 1 .
[0088] The screen 2 is disposed at the through hole 1 a on the supporting plate 1 .
[0089] The suction nozzle 3 is disposed above the supporting plate 1 , and the suction nozzle 3 accommodates the screen 2 therein.
[0090] The capsule chamber 4 is detachably connected under the carrier plate 1, and the capsule chamber is also located at the above-mentioned through hole 1a. The capsule chamber 4 is made of transparent material.
[0091] The button 5 is connected with a pointed pin 6, and the pointed pin 6 can extend into the capsule chamber by pressing the button 5...
Embodiment 2
[0119] Figure 8 and Figure 9 It shows the second preferred embodiment of the dry powder inhaler of the present application, its structure is basically the same as that of the dry powder inhaler in Embodiment 1, the main difference between the two lies in the following aspects:
[0120] First, in this embodiment, the upper shell 9 is no longer an integral structure, but is composed of a split left half shell 901 and a right half shell 902, and the left half shell 901 and the right half shell 902 are pivotally connected respectively On the left side and the right side of the bearing plate 1, the two are split structures.
[0121] Secondly, in this embodiment, the lower case 8 is no longer provided with the notch 8a extending from the upper open edge of the lower case to the bottom of the lower case, and naturally the rotating window 10 with the structure of the first embodiment will not be provided. Instead, this structure is used:
[0122] A U-shaped slit 8c is provided on...
Embodiment 3
[0126] Figure 10 to Figure 18 It shows the third preferred embodiment of the dry powder inhaler of the present application. Its structure is basically the same as that of the dry powder inhaler in Embodiment 1. The main differences between the two lie in the following aspects:
[0127] First, the connection modes among the upper shell 9 , the carrying plate 1 , the suction nozzle 3 and the lower shell 8 are different.
[0128] In this embodiment, the bottom of the above-mentioned suction nozzle 3 is pivotally connected with the bearing plate 1 . Specifically, the pivotal connection of the two is as follows:
[0129] The side of the bearing plate 1 is integrally formed with two bearing plate lugs 1h distributed at intervals, and the inner side of the two bearing plate lugs 1h close to each other is integrally formed with a coaxially arranged cylindrical inner boss 1j. The outer sides of the bearing plate lugs 1h facing away from each other are integrally formed with coaxiall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com