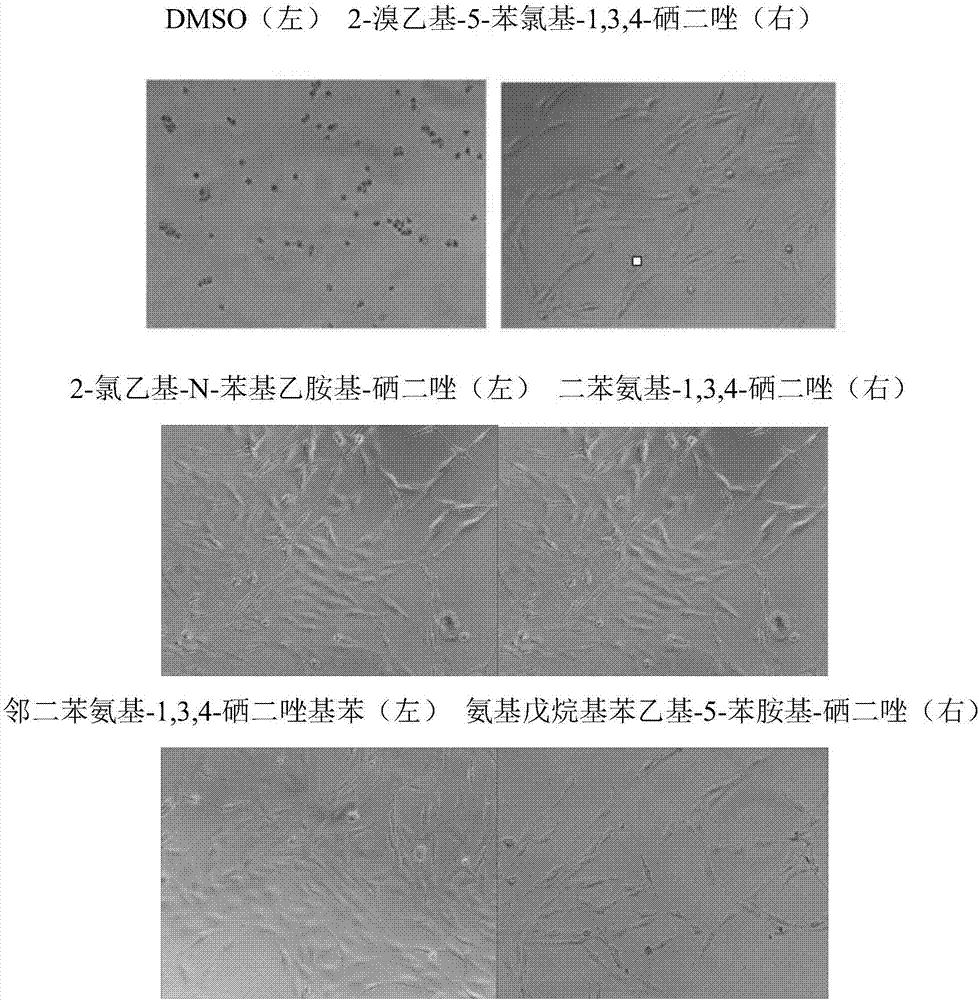
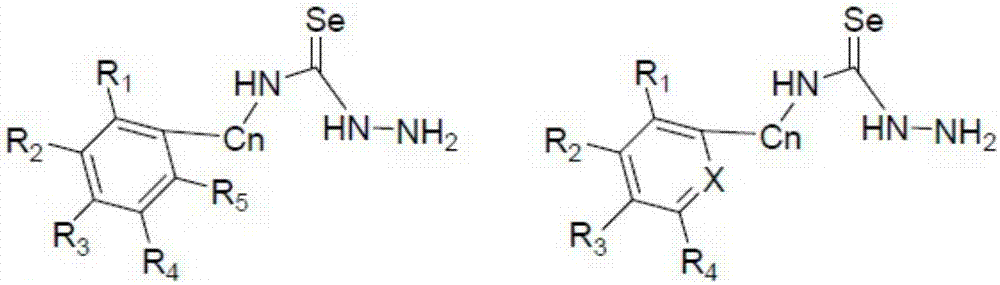
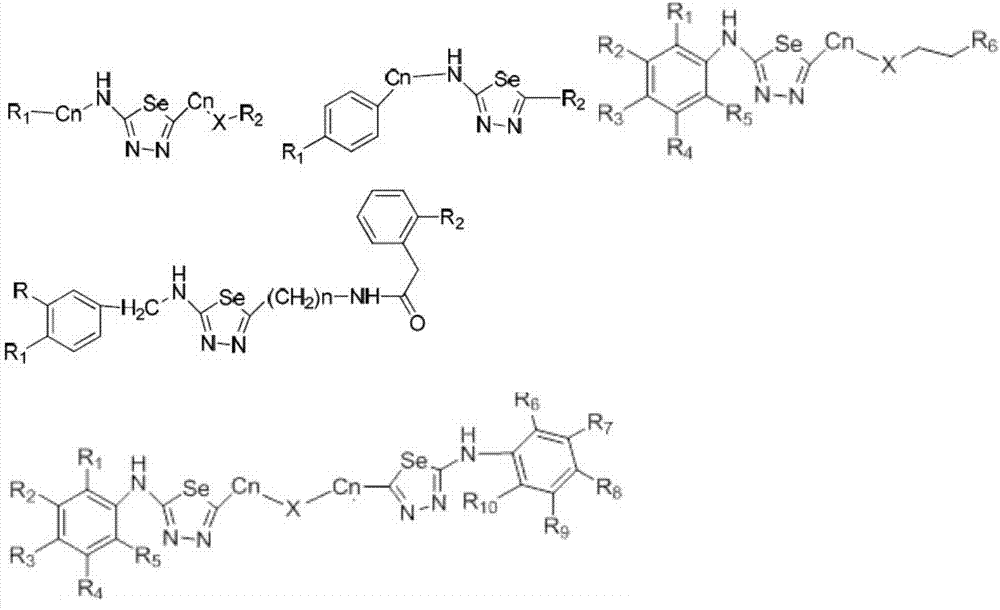
Synthesizing method for 1,3,4-selenadiazole derivative
A synthesis method and derivative technology, which can be used in drug combinations, antidote, antitumor drugs, etc., can solve problems such as unfavorable industrial production and harsh reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] 2-Bromoethyl-N-phenylamino-1,3,4-selenoadiazole:
[0073]
[0074] Substituted selenourea (1mmol; or other selenourea), mixed with polyfunctional carboxylic acid compound (0.2-4mmol), mixed with 5ml POCl3 and stirred evenly; heated to 50-80°C, 5 reacted for 0.5-12h, stopped the oil bath heating, and reacted The solution was slowly poured into ice water, precipitated, separated by column chromatography, and dried; the product was obtained with a yield of 40-95%.
[0075] m / z 332 (100%, M+H + )
[0076] 1 H NMR (500MHz,) δ10.37(s, 1H), 7.65–7.53(m, 2H), 7.35(d, J=9.0Hz, 2H), 6.99(dt, J=7.4, 3.7Hz, 1H), 3.6(t, J=6.5Hz, 2H), 3.38-3.29(t, J=6.5Hz, 2H)
[0077] Experiments were carried out under different reaction conditions to obtain the following table
[0078]
[0079]
[0080] The various reactants and their reaction equations are as follows, and it should be noted that the reaction conditions in the following reactions are all within the reaction conditions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com