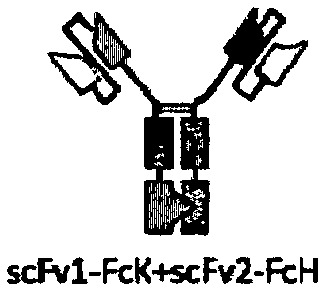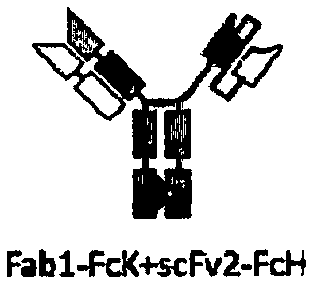Novel bispecific antibody and purpose thereof
A bispecific and antibody technology, applied in the field of new bispecific antibodies and their medical and biological applications, can solve problems such as small molecular weight, inability to mediate biological functions, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1 Preparation and Confirmation of Monoclonal Antibody
[0133] As an example demonstrating the bispecific human IgG1 antibody of the present application, a bispecific human IgG1 antibody targeting human CD3E and HER2 was prepared.
[0134] As the basis for preparing the bispecific human IgG1 antibody, the inventors of the present application first prepared two monoclonal antibodies against human CD3E and HER2. This process required the use of a variety of different recombinant proteins, including the extracellular region (CD3E, SEQ ID NO: 32), human CD3D-extracellular domain (CD3D, SEQ ID NO: 33), monkey CD3E extracellular domain (mfCD3E, SEQ ID NO: 34), monkey CD3D extracellular domain (mfCD3D, SEQ ID NO: 35), mouse CD3E extracellular region (mCD3E, SEQ ID NO: 36), mouse CD3D extracellular region (mCD3D, SEQ ID NO: 37), recombinant HER2 extracellular region D1D2D3 part (HER2, SEQ ID NO :38). In the natural environment, CD3E and CD3D form a heterodimer, so in ...
Embodiment 2
[0138] Example 2 Design and preparation of bispecific antibodies with different structures
[0139] Based on the two monoclonal antibodies prepared and verified in Example 1, a series of bispecific antibodies against human CD3E and HER2 were designed.
[0140] Since each arm of a bispecific antibody can contain Fab fragments or scFv, four structural bispecific antibodies were designed according to different combinations (see Figure 1a-Figure 1d ).
[0141] In addition, in order to promote the formation of heterodimers, Fc fragment mutants (FcK, SEQ ID NO: 41 or FcH, SEQ ID NO: 42) of human IgG1 antibodies based on KIH (Knob-Into-Hole) technology were used, That is, the antigen-binding region for human CD3E (derived from H10B7+L1A7) was fused to the N-terminus of Fc (FcK) containing Knob mutation, and the antigen-binding region for HER2 (derived from C6G9+L1A7) was fused to the N-terminus of Fc (FcK) containing mutation of Hole N-terminus of Fc(FcH).
[0142] According to th...
Embodiment 3
[0147] Example 3 Affinity Analysis of Various Bispecific Antibodies
[0148] Antibody affinity determination was performed with GE's Biacore X100plus. Amine coupling kit (Amine coupling kit), human antibody capture kit (human antibody capture kit), His capture kit (his capture kit), CM5 chip and pH7.4 10×HBS-EP and other related reagents and consumables were purchased from GE Healthcare.
[0149] The affinities of different bispecific antibodies were determined using a capture method. When measuring the affinity of the monoclonal antibody or bispecific antibody prepared in Example 2 to CD3E, the anti-His antibody was coupled to the surface of the CM5 chip, and the CD3E antigen with His tag (CD3E-FcK-His / CD3D -FcH) was captured on the surface of the CM5 chip as a stationary phase, and a series of concentration gradients of various anti-CD3E antibody proteins were set to flow through the surface of the stationary phase by a single cycle method, and the affinity of each antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com