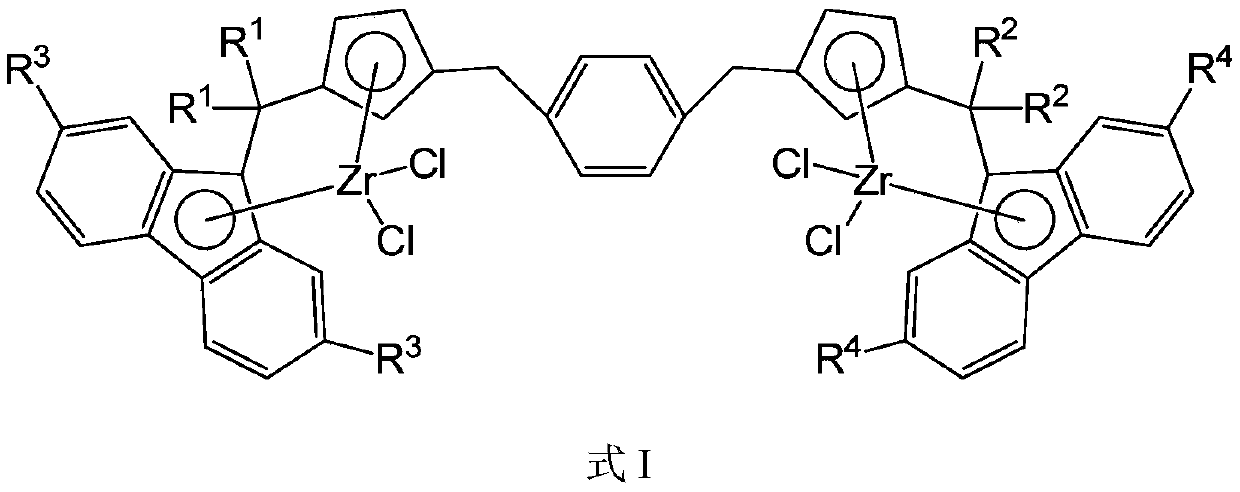
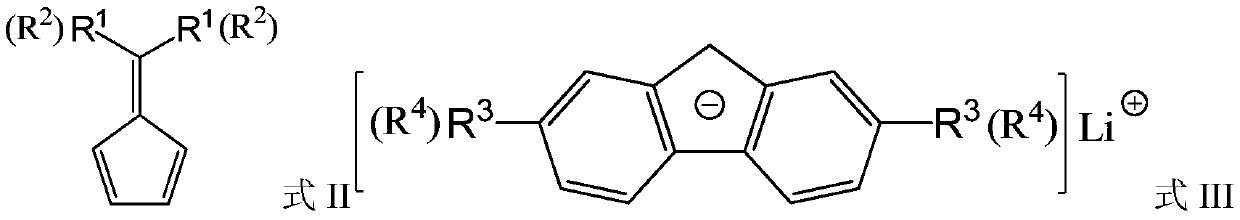
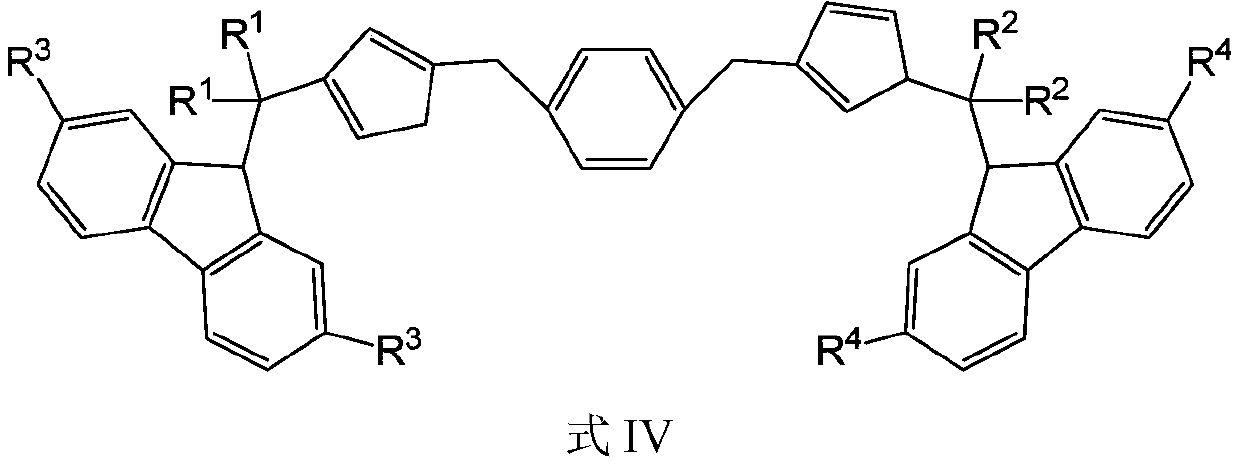
A bridged asymmetric binuclear metallocene compound and its preparation and application
A metallocene compound, asymmetric technology, applied in the direction of metallocene, catalytic reaction, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of molecular weight reduction, achieve stable steric configuration, and facilitate the selection of propylene Sexual polymerization, high activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Ligand L1[(CH 3 ) 2 C(C 5 h 4 )(C 13 h 9 )](CH 2 C 6 h 4 CH 2 )[(C 6 h 5 ) 2 C(C 5 h 4 )(C 13 h 9 )]Synthesis
[0100]
[0101]Under argon protection, with carbon tetrachloride as a solvent, p-xylene (5.04g, 47.53mmol), NBS (30g) and BPO (dibenzoyl peroxide) (2g) were added to the bottle for reaction, heated , when heated to 70°C, the solid and liquid in the bottle began to melt, after stabilization, continue heating to 80°C, reflux reaction for 20 hours, cooling down, white solid in the upper layer, light yellow clear liquid in the lower layer, cooled to 40°C, Filter, take the filtrate, and put it in the refrigerator. After 24 hours, white crystals, about 5 g, are precipitated, which is p-dibromobenzyl, and the yield is 40%.
[0102] Add fluorene (10g, 60.24mmol), 100mL of n-hexane, and 20mL of diethyl ether into a 200mL Schlenk bottle, and slowly add n BuLi (36.7mL, 1.64mol / L), a precipitate formed immediately. Naturally rise to room temperature,...
Embodiment 2
[0113] Ligand L2[(CH 3 ) 2 C(C 5 h 4 )(C 13 h 9 )](CH 2 C 6 h 4 CH 2 )[(CH 2 ) 5 C(C 5 h 4 )(C 13 h 9 )]Synthesis
[0114]
[0115] 0°C, a solution of 6,6-dimethylfulvene (5.11g, 48.18mmol) in ether (40mL) was added dropwise to a solution of fluorenelithium (FluLi) (8g, 48.18mmol) in ether (80mL), after 8h Precipitation appeared, and the reaction was continued for 20 hours, a large amount of precipitates formed, press-filtered, the solid was washed twice with ether, once with hexane, and dried to obtain 9.18 g of solid, ie, the yield was 70%.
[0116] At 0°C, a solution of 6,6-pentamethylenefulvene (8.89g, 48.18mmol) in ether (40mL) was added dropwise to a solution of FluLi (8g, 48.18mmol) in ether (80mL). No precipitate was formed, but after about 4 hours, a precipitate appeared, and after 20 hours of reaction, a large amount of precipitate formed, and the solid was washed twice with ether and once with hexane, and dried to obtain 11.49 g of solid, with a y...
Embodiment 3
[0125] Ligand L3[(C 6 h 5 ) 2 C(C 5 h 4 )(C 13 h 9 )](CH 2 C 6 h 4 CH 2 )[(CH 2 ) 5 C(C 5 h 4 )(C 13 h 9 )]Synthesis
[0126]
[0127] At 0°C, a solution of 6,6-pentamethylenefulvene (8.89g, 48.18mmol) in ether (40mL) was added dropwise to a solution of FluLi (8g, 48.18mmol) in ether (80mL). No precipitate was formed, but after about 4 hours, a precipitate appeared, and after 20 hours of reaction, a large amount of precipitate formed, and the solid was washed twice with ether and once with hexane, and dried to obtain 11.49 g of solid, with a yield of 68%.
[0128] At 0°C, a solution of 6,6-diphenylfulvene (11.08g, 48.18mmol) in ether (40mL) was added dropwise to a solution of FluLi (8g, 48.18mmol) in ether (80mL). After 6 hours, Precipitation appeared, and a large amount of precipitates formed after 20 hours of reaction. The solvent was removed by pressure filtration, and the solid was washed twice with ether and once with hexane, and dried to obtain 12.41 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com