Preparation method of iloperidone intermediate
A technology of iloperidone and intermediates, which is applied in the field of iloperidone products and can solve problems such as inability to remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of (2,4-difluorophenyl)-(4-piperidinyl)methanone oxime hydrochloride
[0044] Add 70g of 4-(2,4-difluorobenzoyl)piperidine hydrochloride into 840mL of ethanol, stir and add 70g of hydroxylamine hydrochloride and 80ml of triethylamine, and heat to reflux for about 1h. Cool to room temperature, filter with suction, wash with a small amount of ethanol, and dry to obtain 55 g of white solid.
Embodiment 2
[0045] Example 2: Preparation of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride
[0046] Add 27g of potassium hydroxide to 600mL of methanol, add 55g of (2,4-difluorophenyl)-(4-piperidinyl)methanone oxime hydrochloride, and heat to react for about 2.5h. Cool to room temperature, add an appropriate amount of anhydrous MgSO4, and stir for about 1 h. Suction filtration, and the filtrate was concentrated under reduced pressure. Add 500 mL of acetone, stir at room temperature for about 0.5 h, filter, add hydrochloric acid dropwise to the filtrate with stirring to make the pH = 2-3, filter with suction, and dry to obtain 35 g of a white solid. The water content in methanol is 0.4%.
Embodiment 3
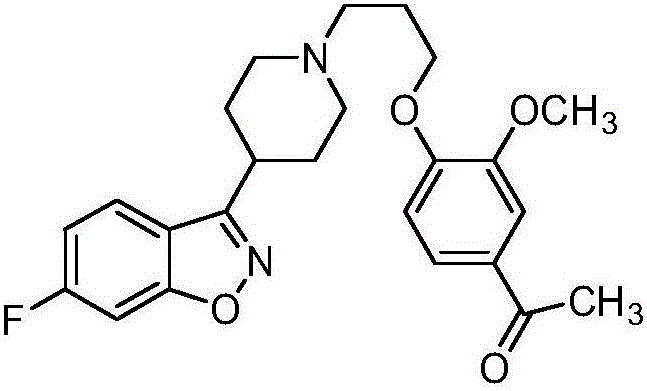
[0047] Embodiment three: the preparation of iloperidone
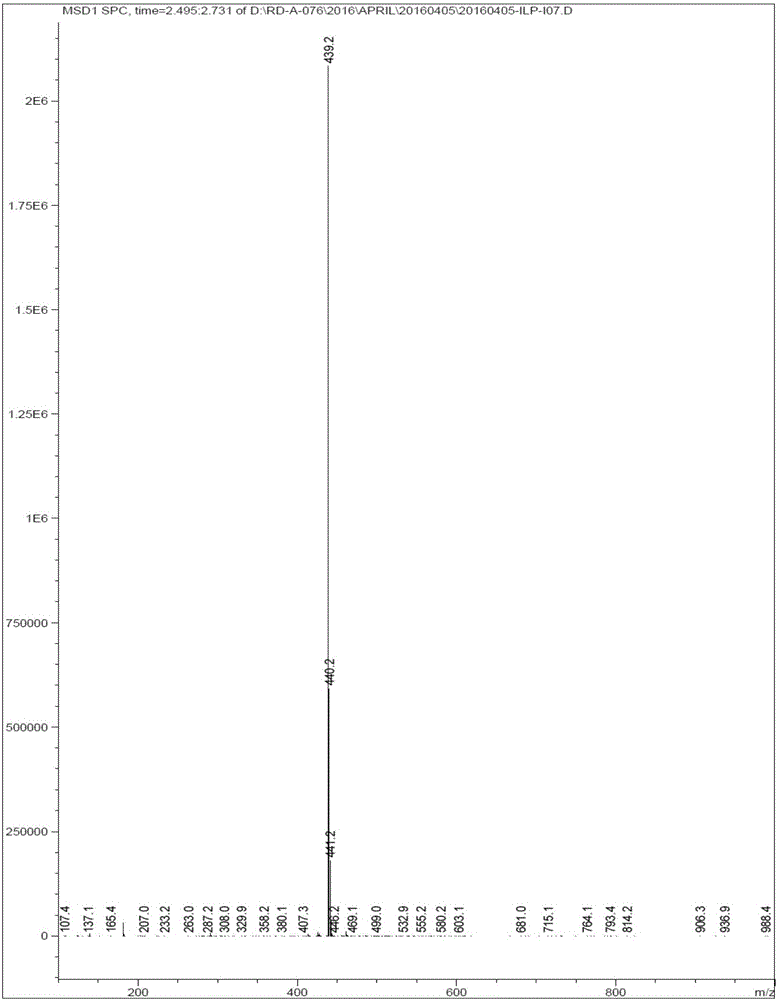
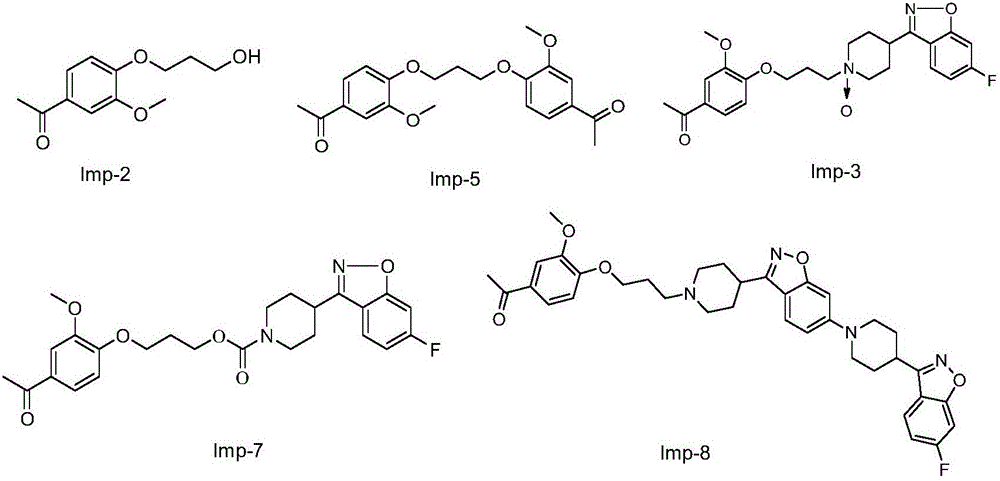
[0048] Add 400mL DMF to 32g of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, add 35g of potassium carbonate, 3.5g of potassium iodide and 3-methoxy-4-( 33.5 g of 3-chloropropoxy) acetophenone, heated for about 7 hours. Cool to room temperature, filter with suction, pour the filtrate into 1000 mL of cold water with stirring, stir for about 2 hours, filter with suction, wash with water, and dry to obtain 53 g of a light yellow crude product. After recrystallization from ethanol, 28 g of iloperidone was obtained, and the content of ILPI-07 was detected to be 0.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com