A kind of isatin derivative and its synthetic method
A synthetic method and derivative technology, applied in the direction of organic chemistry, can solve the problem of lower yield of target product, achieve the effect of reducing by-product generation, increasing yield, and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
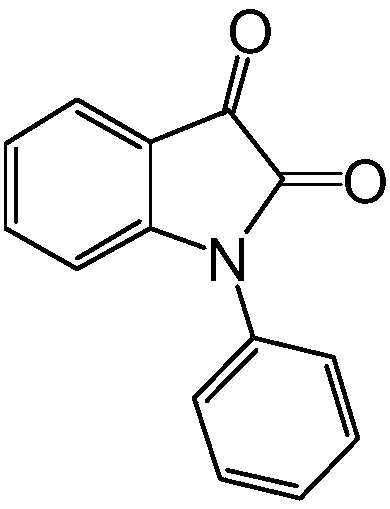
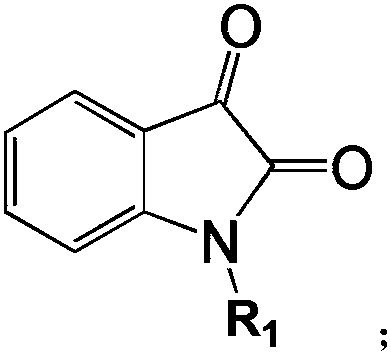
[0033] when R 1 When it is phenyl, product name: N-phenylisatin
[0034]
[0035] Weigh N-phenylindolinone 1mmol (209mg) into a 100mL round-bottomed flask, measure 15ml of dioxane with a graduated cylinder and add it to the round-bottom flask, then add 1mmol (111mg) of selenium dioxide ( SeO 2 ), heated to reflux in an oil bath at 130 °C, and the reaction system continued for 10 h after heating and cooling to room temperature.
[0036] Subsequently, dioxane in the reaction system was removed by rotary evaporation, and 20 ml of ethyl acetate was added to the reaction system to dissolve the target product, followed by extraction twice with a separating funnel, the organic phases were combined, and anhydrous magnesium sulfate was added to dry. Finally, silica gel was added and spun into dry powder and applied to the column, and the mixture of ethyl acetate and petroleum ether was passed through the column to obtain 200.7 mg of N-phenylisatin as a brick-red solid with a yield...
Embodiment 2
[0041] when R 1 When it is methyl, product name: N-methylisatin
[0042]
[0043] In the experiment, the previously added N-phenylindolinone was replaced with N-methylindolinone, and the remaining steps were the same as in Example 1 to obtain a red solid with a yield of 84%.
[0044] 1 H NMR (400MHz, CDCl 3 )δ7.70–7.55(m,1H),7.14(t,J=7.5Hz,1H),6.91(d,J=7.9Hz,1H),3.26(s,1H). 13 C NMR (101MHz, CDCl 3 )δ183.35(s), 158.25(s), 151.47(s), 138.43(s), 125.26(s), 123.84(s), 117.45(s), 109.95(s), 26.22(s).
Embodiment 3
[0046] when R 1 When it is ethyl, product name: N-ethylisatin
[0047]
[0048]In the experiment, the previously added N-phenylindolinone was replaced with N-ethylindolinone, and the remaining steps were the same as in Example 1 to obtain a red solid with a yield of 85%.
[0049] 1 H NMR (400MHz, CDCl 3 )δ7.66–7.55(m,2H),7.12(td,J=7.6,0.5Hz,1H),6.95(d,J=7.9Hz,1H),3.79(q,J=7.2Hz,2H), 1.32(t,J=7.2Hz,3H). 13 C NMR (101MHz, CDCl 3 )δ183.96(s), 158.12(s), 150.88(s), 138.71(s), 125.61(s), 123.89(s), 117.80(s), 110.39(s), 35.20(s), 12.75( s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com