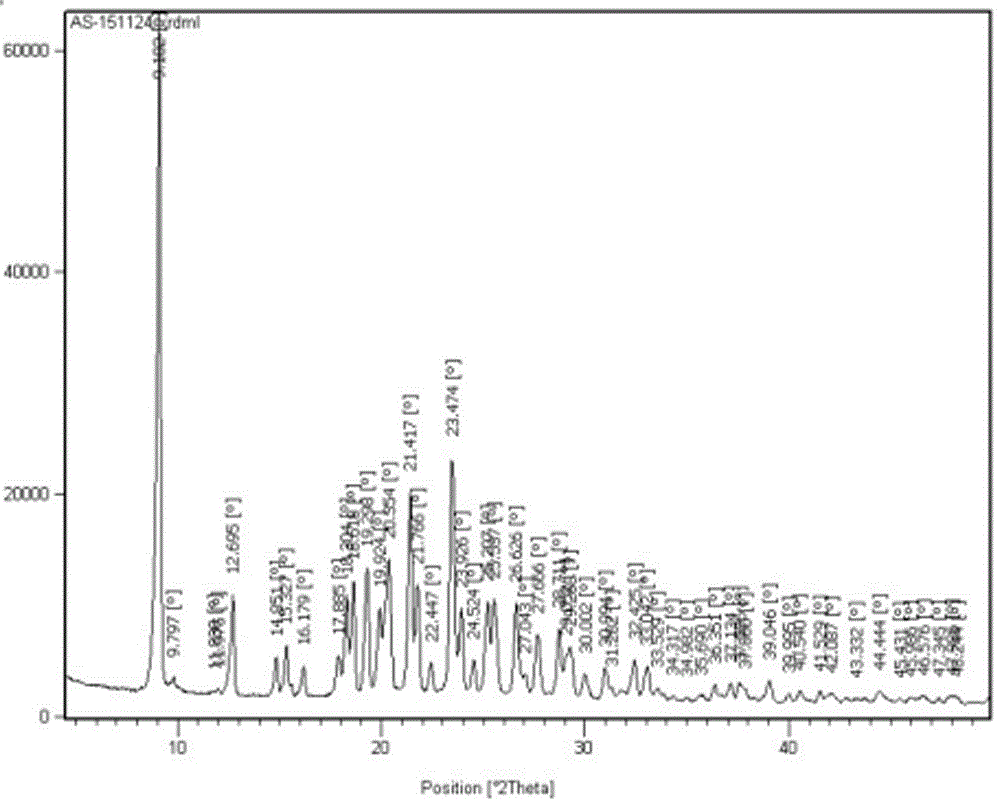
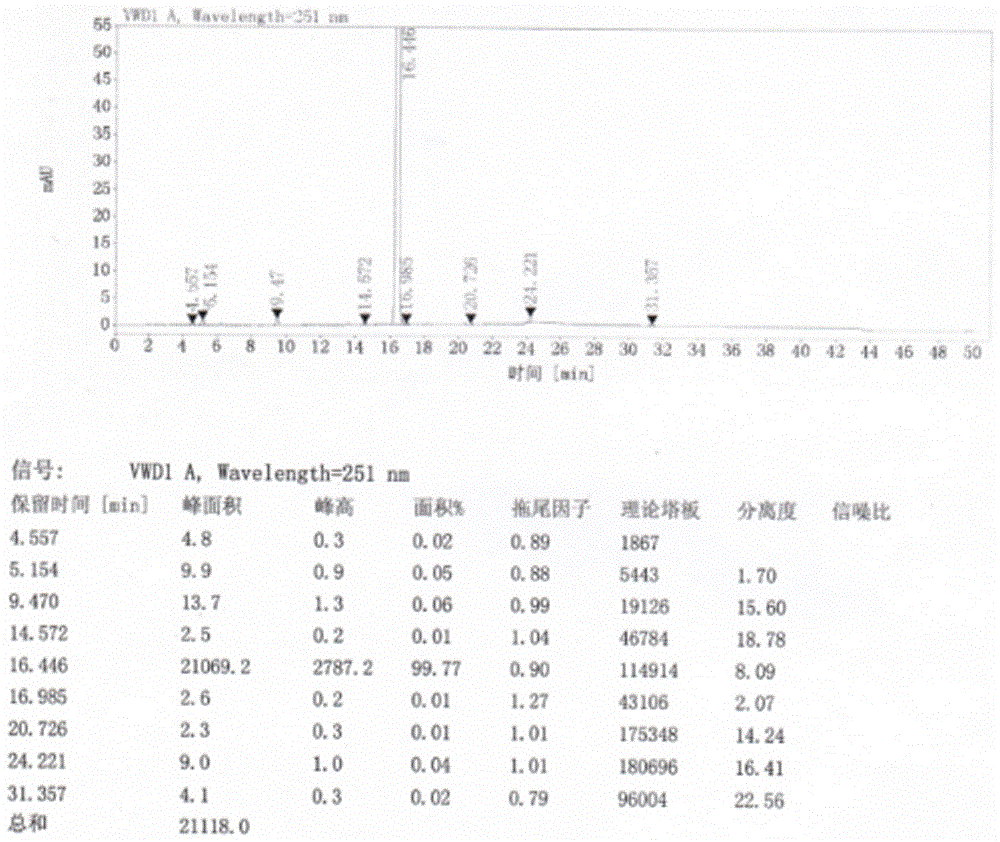
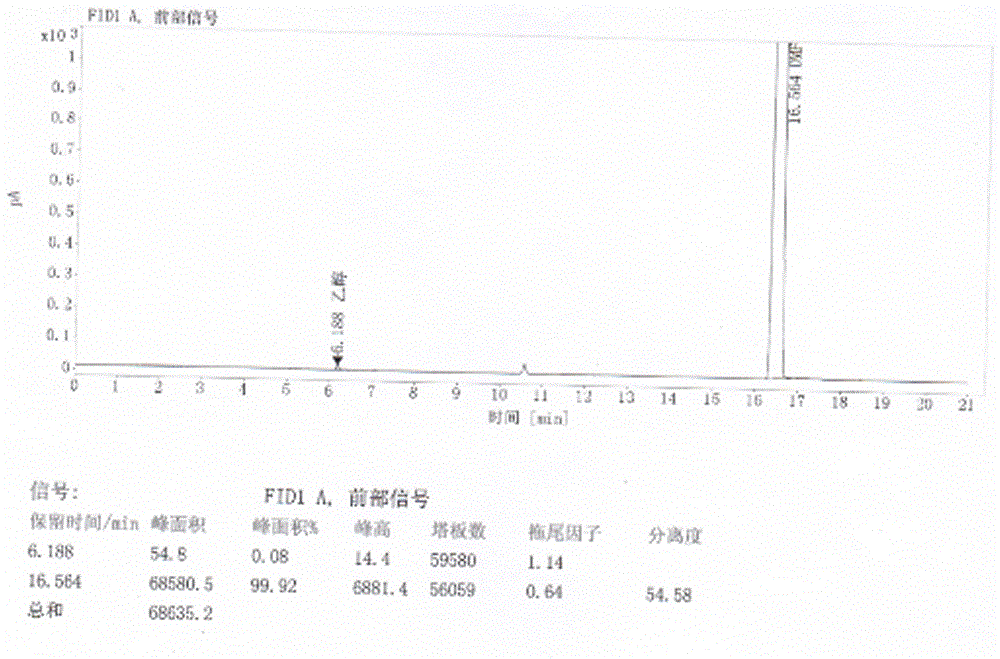
Refining method of crystal type A azilsartan
A refining method and crystal form technology, which is applied in the field of medicine, can solve the problems of large amount of refining solvent, long drying time, and increase of related substances, and achieve the effects of lowering the refining dissolution temperature, reducing production costs, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Put 1kg of ethyl acetate and 3kg of absolute ethanol into a glass reactor and mix them. After heating the temperature to 50-55°C, add 1.3kg of azilsartan crude product, and control the temperature at 50-55°C and stir until the solution is clear.
[0028] Rapidly lower the temperature to 20-25°C, and maintain this temperature to stir and crystallize. Suction filtration, the filter residue is sieved and granulated by a swing granulator, the screen is 24 mesh, and then put into the boiling dryer, and the feeding port is closed. Set the air intake of the boiling dryer to 2800-3000m 3 / h, dry at room temperature for 50min, then gradually increase temperature at 38°C and dry for 70min, particle size control D(90): 300~400μm, dry at 50℃ for 2~3h, particle size control D(90): 100~200μm, material cool to 20±5 °C, 1.25 kg of finished product of azilsartan was obtained, the yield was 96%, the crystal form was A crystal form, the purity was 99.81%, and the residual solvent (ethano...
Embodiment 2
[0030] Put 10kg of ethyl acetate and 40kg of absolute ethanol into the refining tank to mix, and heat to 50-55°C. Add 14.29kg of azilsartan crude product, control the temperature at 50-55°C and stir until the solution is clear.
[0031] Pressure filtration to the crystallization tank, after the pressure filtration is completed, quickly cool down to 20-25°C, and maintain this temperature to stir and crystallize. Filtration, the filter residue is sieved and granulated by a swinging granulator, the screen is 24 mesh, put into the boiling dryer, and the feeding port is closed. Set the air intake to 2800~3000m 3 / h, dry at room temperature for 60min, then gradually increase the temperature at 42°C and dry for 50min, particle size control D(90): 300~400μm, dry at 50℃ for 2~3h, particle size control D(90): 100~200μm, cool the material to 20±5 °C, 13.29 kg of finished product of azilsartan was obtained, the yield was 93%, the crystal form was A crystal form, the purity was 99.82%, a...
Embodiment 3
[0033] Put 100kg of ethyl acetate and 200kg of absolute ethanol into the refining tank to mix, and heat to 50-55°C. Add 90.9kg of azilsartan crude product, control the temperature at 50-55°C and stir until the solution is clear.
[0034] Pressure filtration to the crystallization tank, after the pressure filtration is completed, quickly cool down to 20-25°C, and maintain this temperature to stir and crystallize. Filtration, the filter residue is sieved and granulated by a swinging granulator, the screen is 24 mesh, put into the boiling dryer, and the feeding port is closed. Set the air intake to 2800~3000m 3 / h, dry at room temperature for 60min, then gradually increase the temperature at 40℃ and dry for 60min, particle size control D(90): 300~400μm, dry at 50℃ for 2~3h, particle size control D(90): 100~200μm, cool the material to 20±5 °C, 85.45 kg of finished product of azilsartan was obtained, with a yield of 94%, crystal form A, a purity of 99.77% (ie the content of relate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com