Method for preparing maleic anhydride by oxidation of n-butane
A process method, n-butane technology, applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of reducing the effective volume of the reactor, reducing the production efficiency, prolonging the stable period, etc., and achieves a smooth reaction hot spot The sharp rise of , to ensure stability, the effect of small reaction heat release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] (2) Preparation of vanadium phosphorus oxygen catalyst
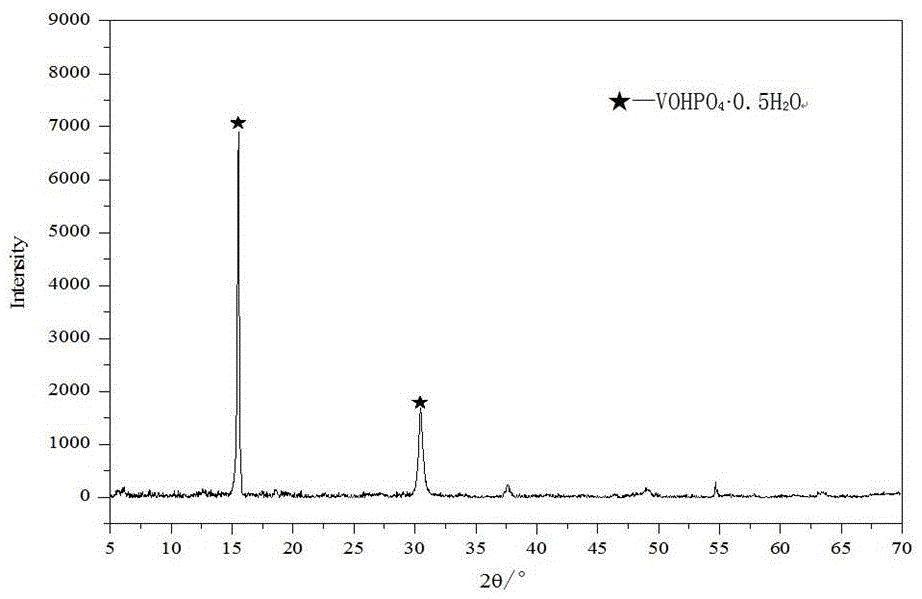
[0052] The vanadium phosphorus oxide obtained in step (1) is first shaped, and the shape of the obtained vanadium phosphorus oxygen catalyst can be a compressed tablet, a spherical shape, an extruded rod, etc., and the phase of the obtained catalyst precursor is (VOHPO 4 0.5H 2 o).
[0053] (3) Oxidation treatment of vanadium phosphorus oxygen catalyst
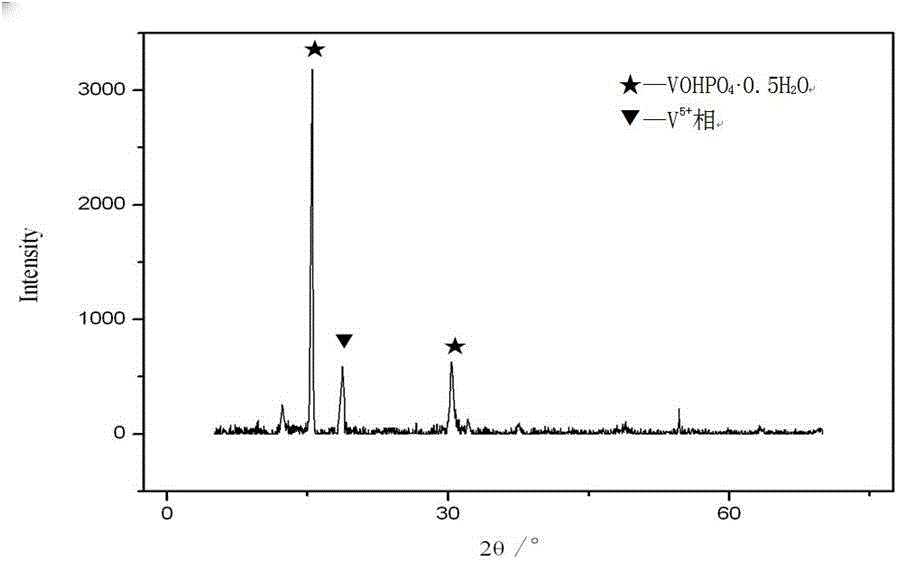
[0054] The catalyst particles obtained in step (2) are immersed in an organic solvent containing a certain concentration of organic peroxide, and the average valence state of vanadium in the catalyst is regulated by controlling the concentration of the organic peroxide.
[0055] The mass concentration of organic peroxides is generally controlled at 0.1% to 10%.
[0056] (4) Activation of vanadium phosphorus oxygen catalyst
[0057] After the impregnation in step (3), it is filtered and dried, and the dried catalyst is activated in a nitrogen or inert gas atmos...
Embodiment 1
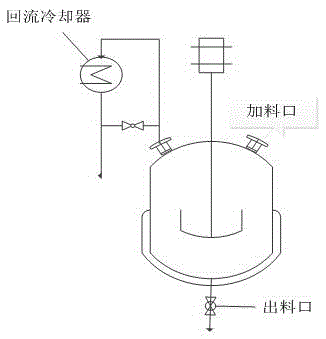
[0063] exist figure 1Add 649L of isobutanol and benzyl alcohol mixture to the reaction kettle shown with a stirring device and a reflux condensing device, the volume ratio of isobutanol / benzyl alcohol is 10:1, 29.53 kg of vanadium pentoxide, and the auxiliary agent hexahydrate Ferric nitrate 0.3kg, auxiliary agent zirconium nitrate 0.5kg, start stirring, increase the reaction temperature and keep it at 100±2°C, carry out reflux reaction, keep reflux time for 4 hours, then add 34.98kg of phosphoric acid with a concentration of 100%, phosphorus / vanadium The molar ratio was 1.1, the reflux was continued for 4 hours, and the reaction ended. After the reaction solution was cooled to room temperature, it was vacuum filtered, and the filter cake was rinsed three times with a small amount of isobutanol to complete the reaction. After the reaction solution was cooled to room temperature, it was vacuum filtered, and the filter cake was rinsed with a small amount of isobutanol three ti...
Embodiment 2
[0068] exist figure 1 Add 649L of isobutanol and benzyl alcohol mixture to the reaction kettle shown with a stirring device and a reflux condensing device, the volume ratio of isobutanol / benzyl alcohol is 10:1, 29.53 kg of vanadium pentoxide, and the auxiliary agent hexahydrate Ferric nitrate 0.3kg, auxiliary agent zirconium nitrate 0.5kg, start stirring, increase the reaction temperature and keep it at 100±2°C, carry out reflux reaction, keep reflux time for 4 hours, then add 34.98kg of phosphoric acid with a concentration of 100%, phosphorus / vanadium The molar ratio was 1.1, the reflux was continued for 4 hours, and the reaction ended. After the reaction solution was cooled to room temperature, it was vacuum filtered, and the filter cake was rinsed three times with a small amount of isobutanol to complete the reaction. After the reaction solution was cooled to room temperature, it was vacuum filtered, and the filter cake was rinsed with a small amount of isobutanol three t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com