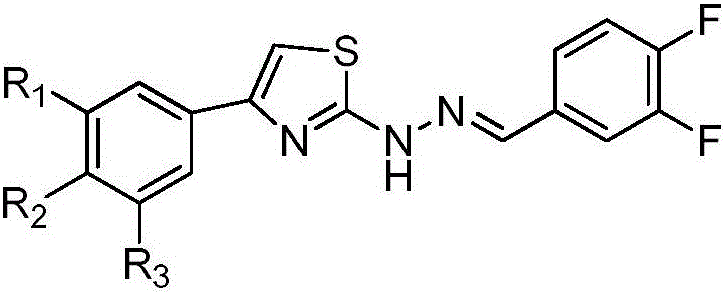
Preparation of novel fluoro thiazole hydrazone compounds and application thereof to anti-tumor medicine
A technology of fluorothiazole hydrazone and substituted thiazole hydrazone, which is applied in the fields of antineoplastic drugs, drug combinations, organic chemistry, etc., can solve the problem of no fluorothiazole hydrazone compounds, etc., and achieve strong inhibitory activity, good application prospects, and good anti-tumor effect. The effect of tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
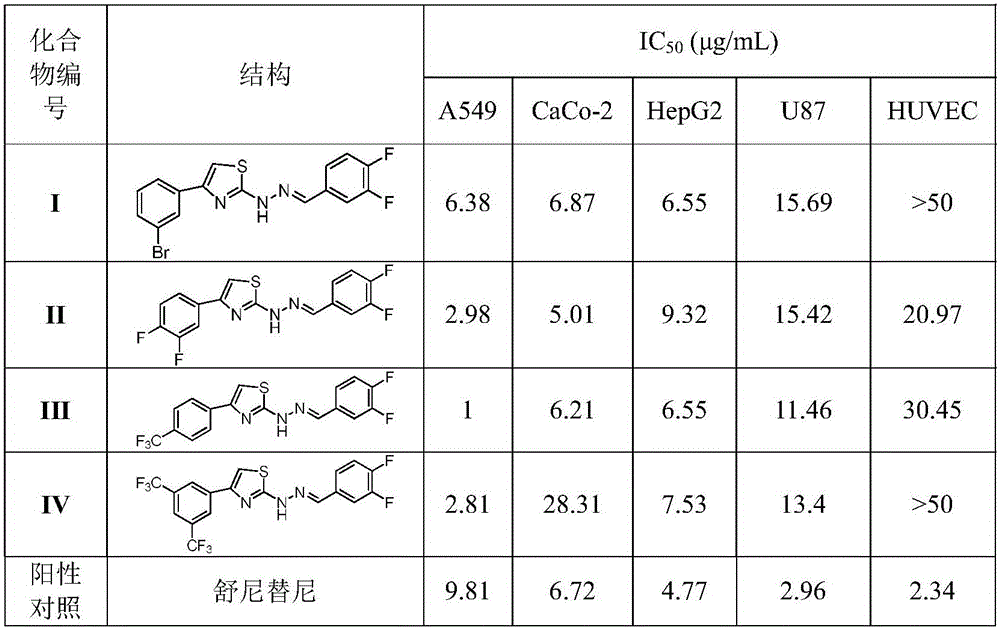
[0026] Example 1: 4-(3-bromophenyl)-2-(2-(3,4-difluorobenzylidene)hydrazino)thiazole (4-(3-bromophenyl)-2-(2-( 3,4-difluorobenzylidene) hydrazinyl) thiazole, compound I) preparation
[0027] (1) Weigh 10mmol 3,4-difluorobenzaldehyde (3.07g) and 11mmol thiosemicarbazide (2.165g) into a 500mL reaction flask, add 30mL ethanol (95%) and stir evenly, then add 1mL glacial acetic acid dropwise , then reflux and stir at 65-70°C for about 10 hours, evaporate most of the ethanol under reduced pressure, add 20 ml of ice water, filter to obtain a precipitate, wash the precipitate with ice water (30 mL for three times), and recrystallize from ethanol to obtain the intermediate 3,4-difluoro Benzylidenethiosemicarbazide (compound a).
[0028] (2) Weigh 1mmol of intermediate 3,4-difluorobenzylidene thiosemicarbazide (215mg) and 4mmol of anhydrous sodium acetate into a 250mL reaction bottle, add 20mL of absolute ethanol, and then add 1mmol of 2,3' -Dibromoacetophenone (278mg), then heated to...
Embodiment 2
[0029]Example 2: 2-(2-(3,4-difluorobenzylidene)hydrazino)-4-(3,4-difluorophenyl)thiazole (2-(2-(3,4-difluorobenzylidene )hydrazinyl)-4-(3,4-difluorophenyl)thiazole, compound II)
[0030] The preparation method of Compound II is similar to that of Compound I, except that the raw material 2,3'-dibromoacetophenone is replaced by 3',4'-difluoro-2-bromoacetophenone from Example 1 Ketone, compound II was prepared, gray solid, yield 82%, 1 H-NMR (500MHz, DMSO- d6 )δ:12.26(1H,s),7.95(1H,s),7.78(1H,m),7.58-7.65(2H,overlap),7.38-7.45(4H,overlap); 13 C-NMR (125MHz, DMSO- d6 )δ: 168.6, 151.1, 150.2, 149.2, 148.8, 148.3, 139.5, 138.7, 132.7, 132.6, 123.7, 122.6, 118.4, 118.1, 114.8, 114.6, 105.4; ESIMS: m / z352[M+H] + HRESIMS: calc for C16H9N3F4S[M+H] + 352.0526,found 352.0452.
Embodiment 3
[0031] Example 3: 2-(2-(3,4-difluorobenzylidene)hydrazino)-4-(4-(trifluoromethyl)phenyl)thiazole (2-(2-(3,4 -difluorobenzylidene)hydrazinyl)-4-(4-(trifluoromethyl)phenyl)thiazole, compound III)
[0032] The preparation method of compound III is similar to the preparation method of compound I, and its difference from Example 1 is that the raw material 2,3'-dibromoacetophenone is replaced by 4'-trifluoromethyl-2-bromoacetophenone , prepared compound III, gray solid, yield 78%, 1 H-NMR (500MHz, DMSO- d6 )δ:12.31(1H,s),8.01(2H,d,J=8.0Hz),7.96(1H,s),7.69(2H,d,J=8.0Hz),7.61(1H,dd,J=9.5 ,9.5Hz),7.50(1H,s),7.38-7.45(2H,overlap); 13 C-NMR (125MHz, DMSO- d6 )δ: 168.8, 151.2, 149.5, 149.3, 139.5, 138.7, 132.6, 128.0, 127.9, 126.4, 125.9, 123.7, 118.3, 114.9, 107.0; ESIMS: m / z 384 [M+H] + HRESIMS:calc forC17H10N3F5S[M+H] + 384.0588,found 384.0535.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com