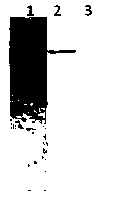Apolipoprotein ii/i and its preparation method, biological function and application
A technology of apolipoprotein and protein, applied in the field of biomedicine, application field in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Isolation and Purification of Natural apoLp-II / I
[0083] 1. Method 1
[0084] Collect the hemolymph, centrifuge at 12000×g and get the supernatant. Carry out 40% ammonium sulfate precipitation; centrifuge to take the precipitate and redissolve it with 50mM citric acid buffer solution pH5.2, 100mM NaCl, 5% glycerol; after centrifugation, take the supernatant, pass through Sephacryl S-200 column, and collect the effluent group containing the target protein After the fraction was dialyzed with 50mM PB pH8.0, it was eluted with a gradient of 0-1M NaCl on a HiTrapTM SP column, and the effluent fraction containing the target protein was collected.
[0085] Test results such as figure 1 -A Swimming lane 7, wherein arrow 1 points to apolipoprotein I, arrow 2 points to apolipoprotein II, and the purity of natural apoLp-II / I reaches electrophoretic purity.
[0086] 2. Method 2
[0087] Tussah hemolymph was precipitated with 70% ammonium sulfate, and then centrifuged at 8000×g ...
Embodiment 2
[0093] Analysis of apoLp-II / I structure and its gene sequence analysis
[0094] According to the techniques, methods and means of conventional protein chemistry and molecular biology, the structure analysis of apoLp-II / I was carried out. The specific methods and means are implemented according to the contents listed in the second content of the invention.
[0095] Obtain the complete nucleotide sequence of apoLp-II / I and its amino acid sequence (as shown in SEQ ID: 1 and ID: 2).
Embodiment 3
[0097] Obtaining Active Fragment of Recombinant apoLp-II / I Using Prokaryotic Expression System
[0098] This example lists and describes the construction strategy and basic method for expressing the apoLp-II / I active fragment gene of the present invention in a prokaryotic expression system.
[0099] The expression vector, expression host cell and expression strategy of the prokaryotic expression system are the conventional and general expression vector, expression host cell and expression strategy of genetic engineering expression.
[0100] This implementation is to enable those skilled in the art to understand the present invention more comprehensively, but not to limit the scope of the special claims of the present invention in any way.
[0101] For the separation and purification method of the expression product, the method, principle, strategy, etc. of Example 1 were adopted.
[0102] 1. Expression vector construction of apoLp-II / I active fragment
[0103] According to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com