A method for extracting lithium from salt lake brine with high selectivity
A salt lake brine, high-selectivity technology, applied in chemical instruments and methods, lithium compounds, inorganic chemistry, etc., can solve the problems of difficult separation, incompatibility, etc., to achieve simple process flow, convenient equipment operation, stable and reliable treatment effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Japan’s Asahi Glass Selemion CSO is selected for the monovalent selective cation exchange membrane, Japan’s Asahi Glass Selemion ASA is selected for the monovalent selective anion exchange membrane, the membrane logarithm is 40, the membrane size is 180mm×550mm, and the effective membrane area is 130mm×390mm. The anode material is iridium plated titanium, and the cathode material is stainless steel. The anolyte is Na with a mass fraction of 3% 2 SO 3 solution, the catholyte is a NaCl solution with a mass fraction of 3%, and its pH is controlled in the range of 2.8 to 3.0 through online adjustment of dilute hydrochloric acid. A brine with a lithium concentration of 0.15 g / L and a Mg / Li mass ratio of 150 was used as the initial desalination solution, and NaCl with a mass fraction of 3% was used as the initial concentrated solution. The linear velocity of the feed liquid on the membrane stack is controlled at 7.1cm / s, and the temperature is controlled at 20°C through a he...
Embodiment 2
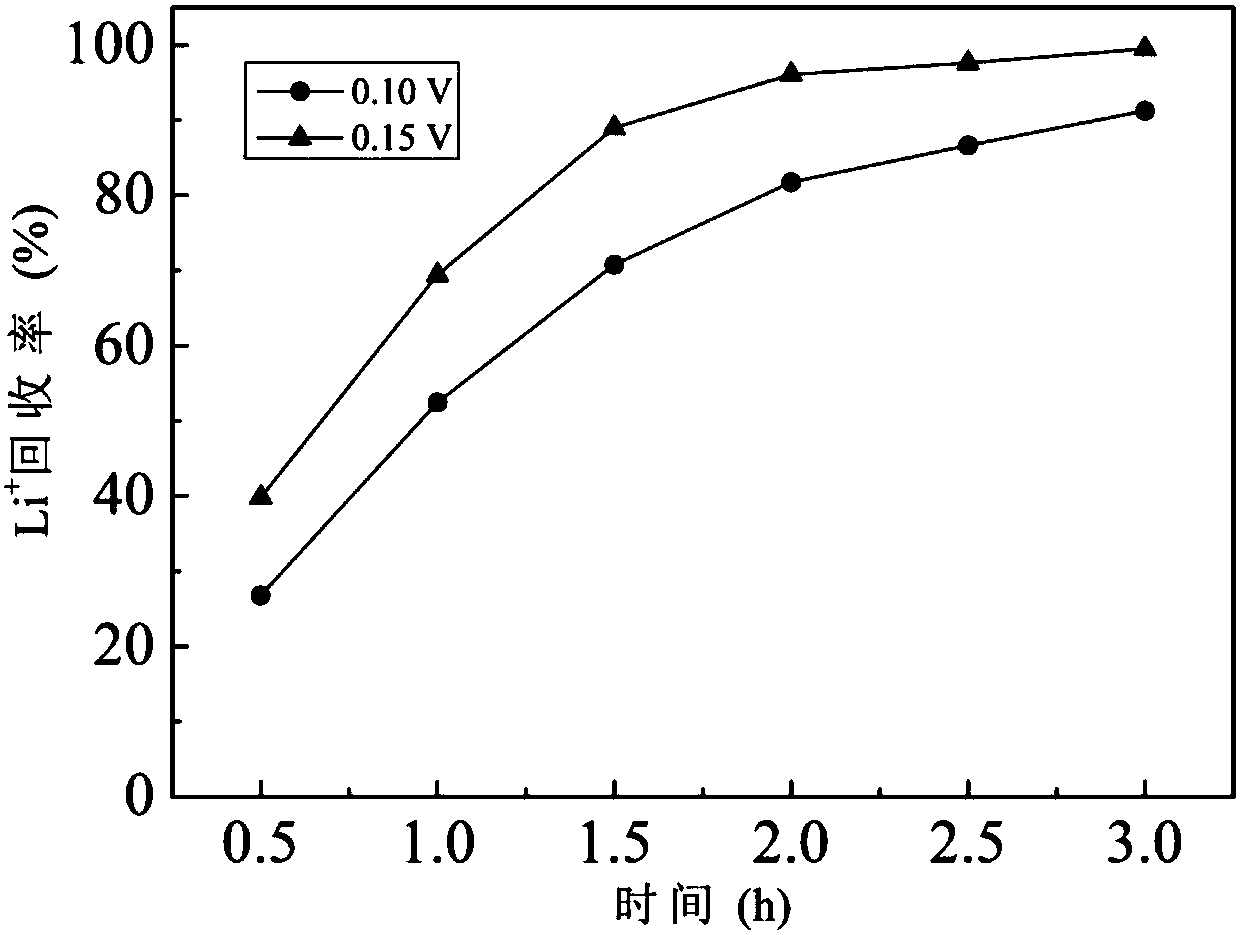
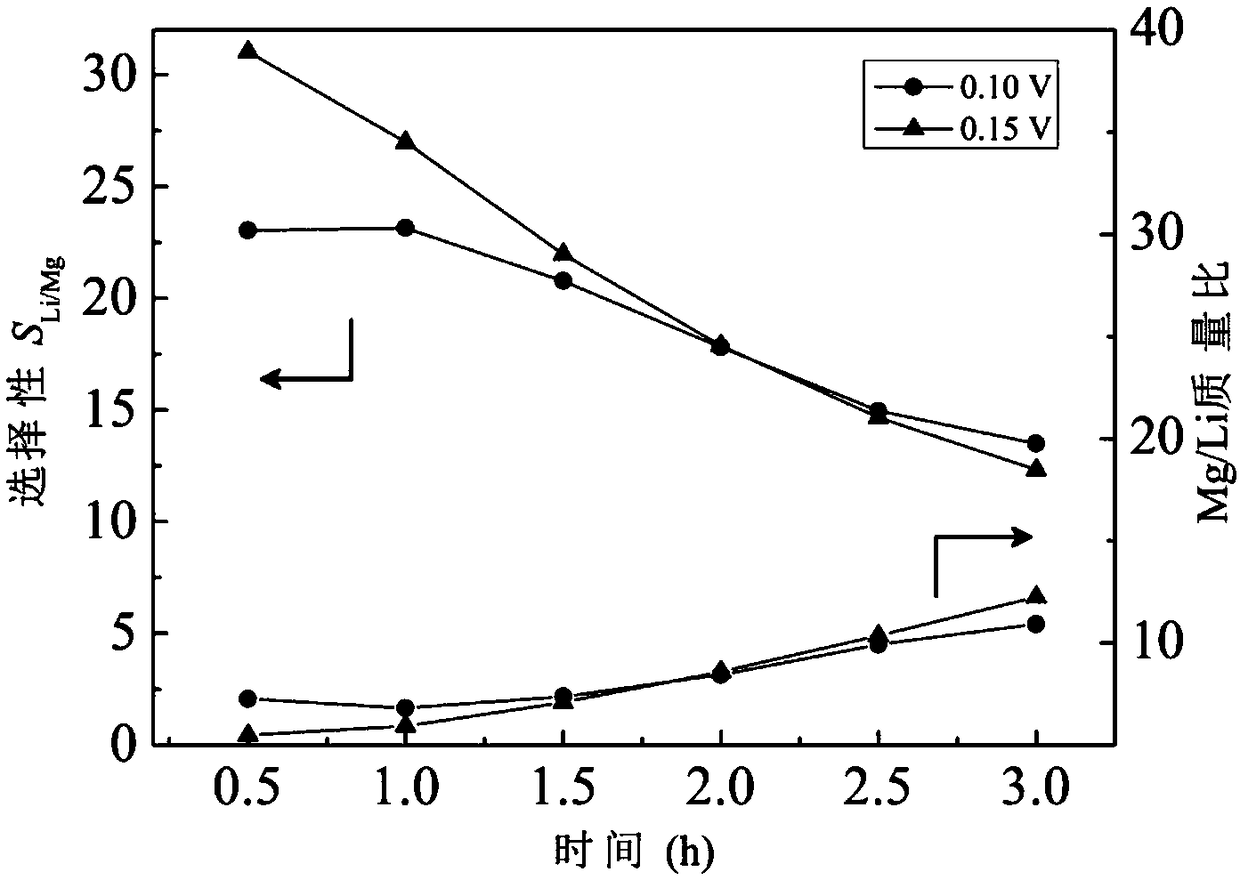
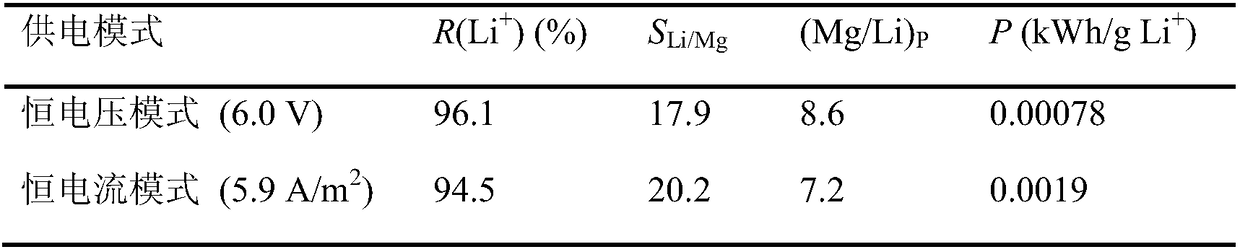
[0043] Raw water quality, polar liquid composition and equipment conditions were the same as in Example 1, and the constant voltage electrodialysis experiment was carried out under the conditions of a single pair of membrane load voltages of 0.10V and 0.15V respectively, and other operating conditions were the same as in Example 1. Li + / Mg 2+ The change of selectivity and product liquid Mg / Li mass ratio with time is shown in the appendix figure 1 , Li + The change of recovery rate with time see figure 2 . in Li + When the recovery rate is about 90%, the separation effect and energy consumption are compared, and the evaluation index comparison is shown in Table 2. The results show that with the increase of operating voltage, the Li + / Mg 2+ The separation selectivity increases significantly, and the Mg / Li mass ratio of the product liquid decreases, while at close Li + Li extracted per unit mass under the recovery rate + The power consumption is equivalent.
[0044] ...
Embodiment 3
[0047] A brine with a lithium concentration of 0.162g / L and a Mg / Li mass ratio of 36.4 was used as the raw material solution, and membrane separation experiments were performed using single-stage electrodialysis and single-stage nanofiltration, respectively, to compare the separation effect and energy consumption. In the electrodialysis experiment, the load voltage of the single membrane was 0.15V, and the composition of the polar liquid, equipment conditions and other operating conditions were the same as in Example 1. The nanofiltration experiment uses the DL-2540 nanofiltration membrane of GE Company, and the nanofiltration membrane separation experiment is carried out under the operating pressure of 0.3-2.1Mpa. The optimal operating pressure is selected, and the separation effect and energy consumption are compared with the electrodialysis method, as shown in Table 3 . The results show that electrodialysis in Li + / Mg 2+ Separation selectivity, product liquid Mg / Li ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com