Compound reserpine tablet for treating hypertension and preparation method thereof
A compound reserpine and high blood pressure technology, which is applied to chemical instruments and methods, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of unsteady dissolution and other problems, and achieve low stirring efficiency and good use stability , strong applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 3.2 parts of reserpine, 310 parts of hydrochlorothiazide, 100 parts of vitamin B6, 100 parts of calcium pantothenate, 300 parts of magnesium trisilicate, 300 parts of potassium chloride, 100 parts of vitamin B1, 420 parts of dihydralazine sulfate, 210 parts of promethazine hydrochloride, 25 parts of ethyl cellulose, 35 parts of corn starch, 15 parts of sodium starch glycolate, and 2 parts of magnesium stearate, take the above-mentioned raw materials and auxiliary materials and put them in a crushing equipment for crushing; Mix evenly, granulate according to conventional methods, compress into tablets, and pack to obtain finished products.
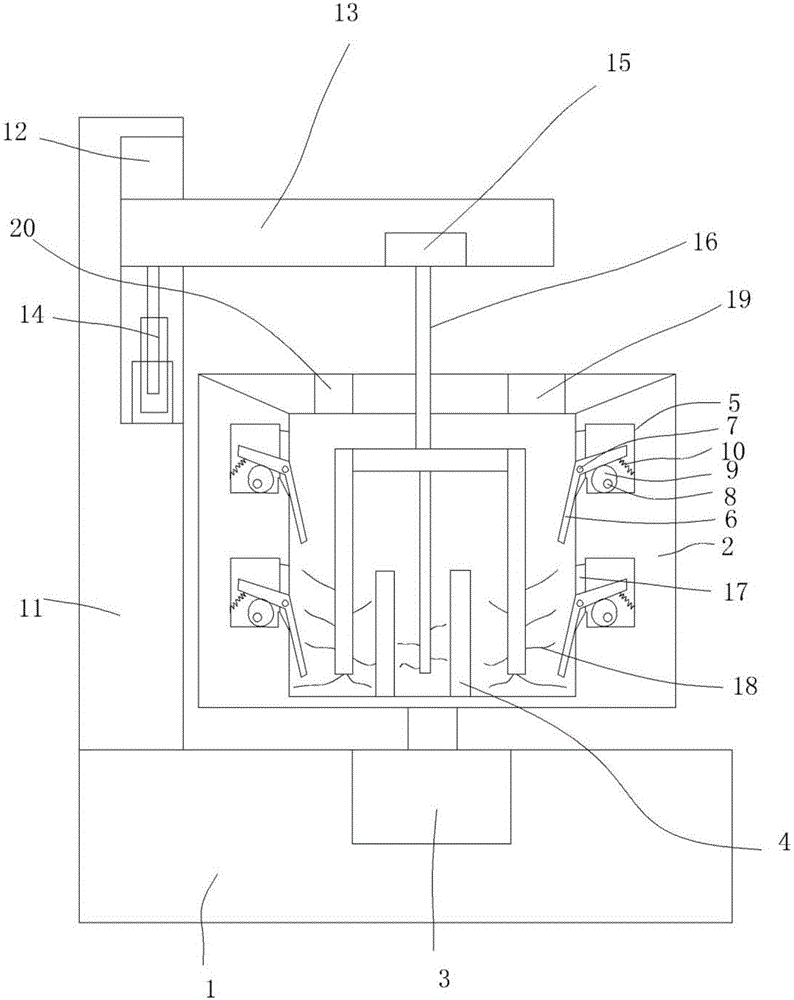
[0017] Particle mixers above, see figure 1 , including a base 1, a mixing cylinder 2 arranged on the base 1, a stirring motor 3 is arranged in the base 1, the main shaft of the stirring motor 3 is in contact with the center of the bottom wall of the mixing cylinder 2 Connected, the bottom wall in the mixing cylinder 2 is provi...
Embodiment 3
[0023] Example 3 Research on the Release of Compound Reserpine Tablets
[0024] Get respectively the tablet that the embodiment of the present invention 1, comparative example 2 prepare, according to the release assay method [2005 version 5 Chinese Pharmacopoeia (two) appendix XD first method], with 0.5% sodium lauryl sulfate solution 1000mL As a solvent, the rotation speed is 50r / min. Operate according to the law. Take 10mL of the solution at 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24h, filter, and replenish 10mL of the above solvent in time Get continued filtrate, measure absorbance at 274nm wavelength according to spectrophotometry (2005 edition 5 Chinese Pharmacopoeia (two) appendix IVA); In addition, accurately weigh the amount of reserpine reference substance that is dried at 105 degrees for 2h, add 0.5% ten The dialkyl sodium sulfate solution was heated and sonicated to dissolve and quantitatively diluted to a solution containing 10ug of drug per 1mL. The absorbance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com