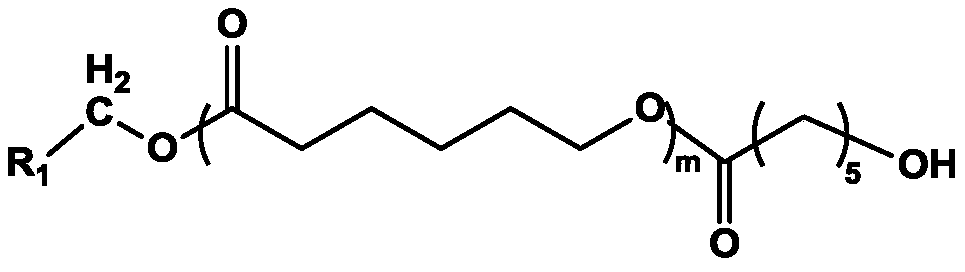
A kind of pelcl/polycaprolactone-g-polyethylene glycol-redv electrospun fiber membrane and preparation method
A polyethylene glycol and caprolactone technology, applied in the field of biomedical materials, can solve the problems of increasing the cost of raw materials and reducing the activity of biologically active substances, and achieve good fiber morphology, good biological activity, and promote the adhesion of vascular endothelial cells and growth effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of polyε-caprolactone-g-polyethylene glycol-REDV is characterized in that comprising steps:
[0036] (1) Amination modification of polyε-caprolactone: prepare polyε-caprolactone into 0.3-0.5g / mL anhydrous dichloromethane solution, and use N,N'-carbonyldiimidazole (CDI) to activate Hydroxyl group, add CDI according to the molar ratio of [single-end hydroxyl polyε-caprolactone] / [CDI] 1:1 (or [double-end hydroxyl polyε-caprolactone] / [CDI] 1:2) , at N 2 Stir and react at room temperature for 24 hours, then add excess ethylenediamine, stir and react at room temperature for 48 to 72 hours, precipitate the product with excess methanol, and dry it in vacuum to obtain the amino-modified product of polyε-caprolactone.
[0037] (2) Preparation of poly-caprolactone-g-polyethylene glycol: prepare the amino-modified poly-caprolactone into a dichloromethane solution with a solubility of 200-300 mg / mL, according to the polyε-caprolactone The molar ratio of the ...
Embodiment 1
[0043] In a three-neck flask equipped with magnetic stirring, 3 g of polyε-caprolactone ( double-terminated hydroxyl) was dissolved in 10mL of anhydrous dichloromethane, according to the molar ratio [double-terminated polyε-caprolactone] / [CDI] was 1:2 to add CDI65.0mg, in N 2 The reaction was stirred at room temperature for 24 hours, then 2.0 mL of ethylenediamine was added, and the reaction was stirred at room temperature for 48 hours. The product was precipitated with excess methanol and dried in vacuum for 24 hours to obtain an amino-modified polyε-caprolactone.
[0044] Dissolve 78 mg of the amino-modified polyε-caprolactone in 3 mL of dichloromethane solution, and the molar ratio of the amino group in the amino-modified polyε-caprolactone to the NHS group in the double-ended PEG is 1 : 1 Add double-ended PEG, maleimide-polyethylene glycol-succinimide acetate (MAL-PEG-NHS, ) 208 mg, stirred at room temperature for 6 h, and the product was precipitated with ethanol to ob...
Embodiment 2
[0047] In a three-neck flask equipped with magnetic stirring, 5 g of polyε-caprolactone ( double-terminal hydroxyl) was dissolved in 10mL of anhydrous dichloromethane, according to the molar ratio [double-terminal hydroxyl polyε-caprolactone] / [CDI] is 1:2, add CDI 32.4mg, in N 2 The reaction was stirred at room temperature for 24 hours, then 2.0 mL of ethylenediamine was added, and the reaction was stirred at room temperature for 60 hours. The product was precipitated with excess methanol and dried in vacuum for 24 hours to obtain an amino-modified polyε-caprolactone.
[0048] Dissolve 600 mg of the amino-modified polyε-caprolactone in 3 mL of dichloromethane solution, and the molar ratio of the amino group in the amino-modified polyε-caprolactone to the NHS group in the double-ended PEG is 1 :1 Add double-ended PEG, maleimide-polyethylene glycol-succinimide acetate (MAL-PEG-NHS, ) 120 mg, stirred at room temperature for 10 h, and the product was precipitated with ethanol t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com