Application of tissue-engineering bone
A tissue-engineered bone, a technology for use, applied in the field of medicine, can solve problems that need to be developed, and achieve the effects of easy access, effective repair and healing, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
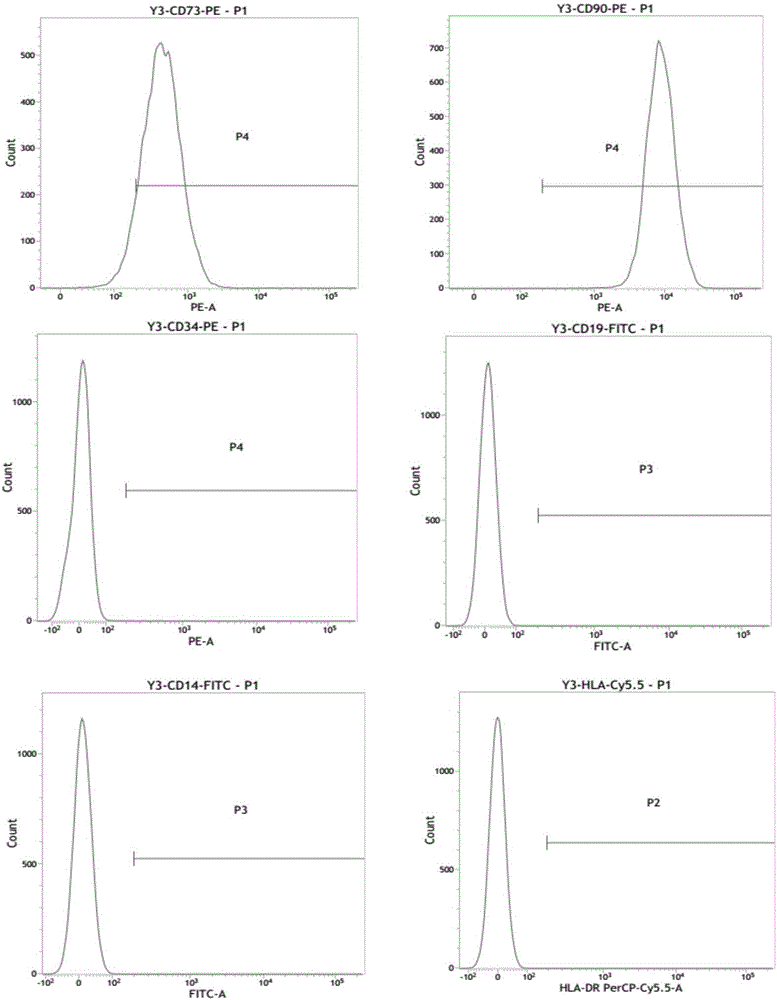
[0048] Example 1 Identification of surface markers of mesenchymal stem cells by flow cytometry
[0049] (1) mesenchymal stem cells (cell shape as figure 1 shown) were cultured in a 10 cm cell culture dish containing serum-free medium (purchased from Sanli Company, China). When the cells grow and reach more than 90% confluency, discard the medium and wash the cells once with PBS buffer, add TrypLe Express enzyme to digest the cells, and when the cells become round and fall off the culture plate, use 10% fetal The αMEM medium of bovine serum was digested, and the cell suspension was collected into a 15ml centrifuge tube, and centrifuged at 1200rpm for 5 minutes. After the supernatant was discarded, the cells were resuspended in fresh serum-free medium, subcultured for 3 generations, and the cells of the third generation were taken and digested with trypsin.
[0050] (2) Put the digested mesenchymal stem cells into a 1.5mL EP tube, add 1 μl flow cytometry antibodies (CD73, CD90...
Embodiment 2
[0054] Example 2 Screening of CD51-expressing mesenchymal stem cells
[0055] (1) Culture mesenchymal stem cells in a 10cm cell culture dish containing serum-free medium. When the cells grow and reach more than 90% confluency, discard the medium and wash the cells once with PBS buffer, add TrypLe Express enzyme to digest the cells, and when the cells become round and fall off the culture plate, use 10% fetal The αMEM medium of bovine serum was digested, and the cell suspension was collected into a 15ml centrifuge tube, and centrifuged at 1200rpm for 5 minutes. After the supernatant was discarded, the cells were resuspended in fresh serum-free medium, subcultured for 3 generations, and the cells of the third generation were taken and digested with trypsin.
[0056] (2) Put the digested mesenchymal stem cells into a 1.5mL EP tube, add 10μl CD51 flow cytometry antibody, and incubate at 4°C in the dark for 30 minutes.
[0057] (3) The cells were washed twice with PBS buffer, and...
Embodiment 3
[0059] Example 3 Comparison of osteogenic differentiation of mesenchymal stem cells+pre-induced cells and mesenchymal stem cells
[0060] (1) When the mesenchymal stem cells screened in Example 2 are cultured to 80-90% confluence, the cells are digested with trypsin and counted, and then counted according to 8000 cells / cm 2 Inoculated into 6-well plates (α-MEM medium containing 10% by volume of fetal bovine serum), and then put the cells in 37°C, 5% CO 2 cultured in an incubator.
[0061](2) On the second day, the osteogenic induction medium was replaced, and the medium components were DMEM, 10% fetal bovine serum, 0.1 mM dexamethasone, 10 mM β-sodium glycerophosphate and 50 μM ascorbic acid.
[0062] (3) The medium was changed once every two days, and the cells were induced for 7 days, and the obtained cells were pre-induced cells.
[0063] (4) After 7 days, discard the medium, rinse the cells once with PBS buffer, add TrypLe Express enzyme to digest the cells, and when the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com