Preparation method for preparing vancomycin from 2-methylpyridine
A vancomycin and picoline technology, applied in the direction of peptides, etc., can solve the problems of difficult crystallization, slow growth of vancomycin hydrochloride crystals, difficult crystallization, etc., and achieves simple production method, improved appearance and color, and effective composition. increased effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
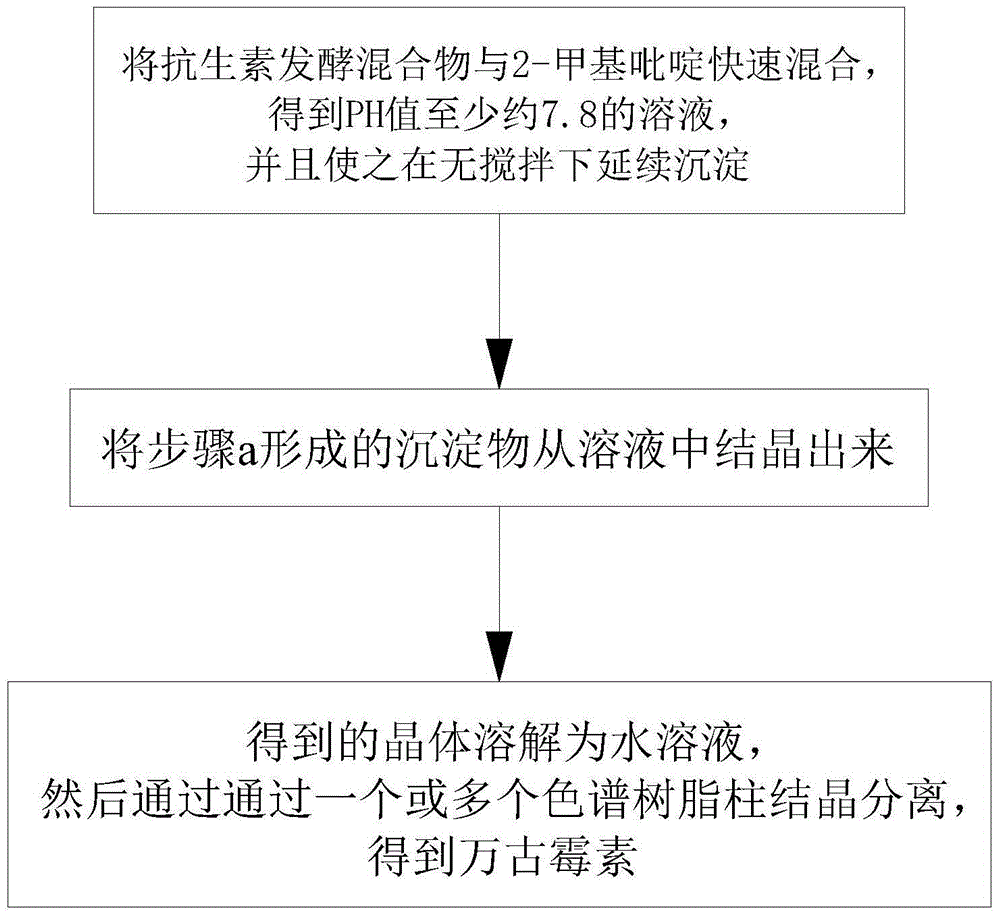
[0020] see figure 1 , the invention provides a kind of preparation method that prepares vancomycin by 2-picoline, comprises the following steps:
[0021] a. Rapidly mixing the antibiotic fermentation mixture with 2-picoline to obtain a solution with a pH value of at least about 7.8, and allowing it to continue to precipitate without stirring;
[0022] b, crystallizing the precipitate formed in step a from the solution;
[0023] c. The obtained crystals are dissolved in an aqueous solution, and then separated by crystallization through one or more chromatographic resin columns to obtain vancomycin.
Embodiment 1
[0025] According to step a, the pH of the 2-picoline is 1.0; according to step b, by adding a water-miscible organic solvent to the precipitate to crystallize, the content of vancomycin in the solution before crystallization is 40%; the The organic solvent is methanol; the added amount of the organic solvent is: methanol is 2 times the volume of the crystallization solution; according to step b, the initial temperature of the crystallization process is 25°C; the end temperature of the crystallization process is 0°C; the cooling rate of the crystallization process is 2 °C / min; according to step c, the concentration of vancomycin after the crystals are dissolved in water is 60%.
Embodiment 2
[0027] According to step a, the pH of the 2-picoline is 2; according to step b, by adding a water-miscible organic solvent to the precipitate to crystallize, the content of vancomycin in the solution before crystallization is 50%; the The organic solvent is methanol; the added amount of the organic solvent is: methanol is 3 times the volume of the crystallization solution; according to step b, the initial temperature of the crystallization process is 35°C; the end temperature of the crystallization process is 10°C; the cooling rate of the crystallization process is 3 °C / min; according to step c, the concentration of vancomycin after the crystals are dissolved in water is 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com