Charge-reversed DNA (Deoxyribose Nucleic Acid) nano-carrier and preparation method and application thereof
A technology of charge reversal and nano-carriers, which is applied in the direction of DNA/RNA vaccination, complete cell/virus/DNA/RNA components, medical preparations of non-active ingredients, etc. Tropical immune response and other issues, to achieve the effect of low cost, good stability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
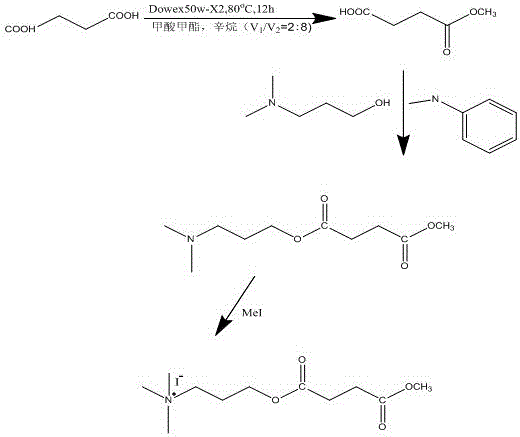
[0046] 1. Preparation of methyl succinate
[0047] Weigh succinic acid (0.118g, 1mmol), Dowex 50W-X 2 (1.0g) was added to 10ml of methyl formate / octane (volume ratio=2:8) mixture, and the reaction was continued in a water bath at 80°C for 12h. The solution was filtered and evaporated to dryness, and the resulting product was purified by chromatography.
[0048] 2. Preparation of 3-dimethylamino-1-propyl methylsuccinate
[0049] Dissolve 0.4 g (20 mmol) of DCC (dicyclohexylcarbodiimide) in 5 ml of dichloromethane,
[0050] Weigh 0.732g (5.5mmol) of methyl succinate in 1, 0.223g (2.5mmol) of 3-dimethylamino-1-propanol, and dissolve 12mg of 4-methylaminopyridine in 15ml of dichloromethane. The DCC was added to the above solution, and the solution was continuously stirred for 2d. The reaction mixture was filtered to remove insoluble matter.
[0051] 3. Preparation of methyl succinic acid-3-dimethylamino-1-propyl ester quaternary ammonium salt
[0052] Weigh 0.347g (1.7mmol) of...
Embodiment 2
[0060] 1. Preparation of methyl succinate
[0061] Weigh succinic acid (0.118g, 1mmol), Dowex 50W-X 2 (1.0g) was added to 10ml of methyl formate / octane (volume ratio=2:8) mixture, and the reaction was continued in a water bath at 80°C for 12h. The solution was filtered and evaporated to dryness, and the resulting product was purified by chromatography.
[0062] 2. Preparation of 3-dimethylamino-1-propyl methylsuccinate
[0063] Dissolve 0.4 g (20 mmol) of DCC (dicyclohexylcarbodiimide) in 5 ml of dichloromethane,
[0064] Weigh 0.732g (5.5mmol) of methyl succinate in 1, 0.223g (2.5mmol) of 3-dimethylamino-1-propanol, and dissolve 12mg of 4-methylaminopyridine in 15ml of dichloromethane. The DCC was added to the above solution, and the solution was continuously stirred for 2d. The reaction mixture was filtered to remove insoluble matter.
[0065] 3. Preparation of methyl succinic acid-3-dimethylamino-1-propyl ester quaternary ammonium salt
[0066] Weigh 0.347g (1.7mmol) of...
Embodiment 3
[0074] 1. Preparation of methyl succinate
[0075] Weigh succinic acid (0.236g, 2mmol), Dowex 50W-X 2 (2.0g), added to 20ml of methyl formate / octane (volume ratio=2:8) mixed solution, 80 ° C water bath reaction continued for 12h. The solution was filtered and evaporated to dryness, and the resulting product was purified by chromatography, namely To obtain methyl succinate;
[0076] 2. Preparation of 3-dimethylamino-1-propyl methylsuccinate
[0077] Dissolve 0.8g (40mmol) of DCC (dicyclohexylcarbodiimide) in 10ml of dichloromethane,
[0078] Weigh 1.464g (11mmol) of methyl succinate in 1, 0.446g (5mmol) of 3-dimethylamino-1-propanol, and dissolve 24mg of 4-methylaminopyridine in 15ml of dichloromethane, and dissolve the prepared DCC Added to the above solution, the solution was continuously stirred for 2d, and the reaction mixture was filtered to remove insoluble matter.
[0079] 3. Preparation of methyl succinic acid-3-dimethylamino-1-propyl ester quaternary ammonium salt ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com