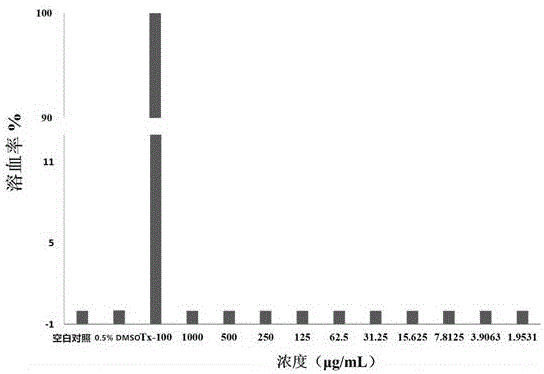
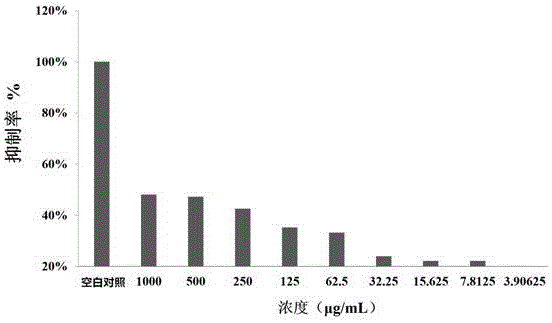
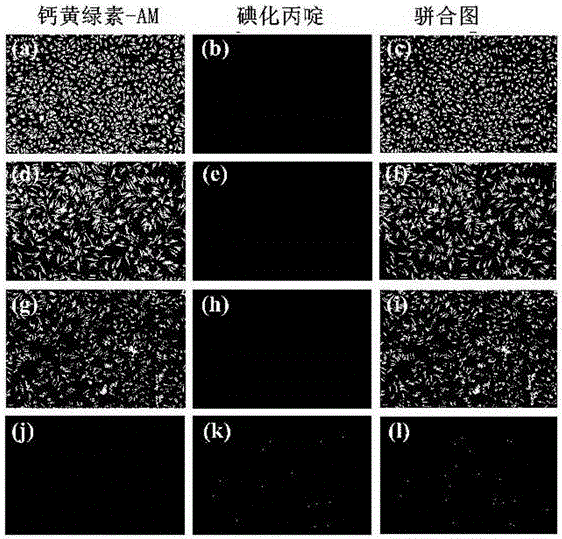
Metal beta-lactamase inhibitor open chain pyridine carboxylic acid derivative and preparation method thereof
An open-chain pyridine carboxylic acid and lactamase technology, which can be applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of unsatisfactory effect of restoring the sensitivity of meropenem and the like, and achieve a simple synthesis method. Feasible, high yield, low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 compound b:
[0025] Take dimethyl 2,6-pyridinedicarboxylate (1.03g, 5.26mmol) in a round bottom flask, add methanol (50mL) under ice-cooling, stir for several minutes, add NaBH in batches 4 (743.80mg, 19.66mmol), connected to a three-way air balloon, and observed by TLC. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and saturated NaHCO 3 solution to remove excess NaBH 4 , then extracted with dichloromethane (3×30mL), back-extracted with water three times, washed with saturated brine twice, and finally added anhydrous MgSO 4 After drying, it was filtered and spin-dried to obtain a white solid (661 mg) with a yield of 75%.
[0026] 1 H NMR (400MHz, CDCl 3 )δ8.21(d, J=7.8Hz, 1H), 7.90(d, J=7.7Hz, 1H), 7.74(t, J=7.8Hz, 1H), 7.51(d, J=7.8Hz, 1H) ,7.25(s,1H),4.36(s,1H),3.93(s,1H),3.88(s,3H),1.17(s,2H),0.91–0.63(m,1H). 13 C NMR (101MHz, CDCl 3 )δ165.53(s), 160.28(s), 146.95(s), 137.66(s)...
Embodiment 2
[0027] The preparation of embodiment 2 compound c:
[0028] Under ice-bath conditions, compound b (500mg) was added into chloroform (10mL), stirred and dissolved, then slowly added dropwise PBr 3 (341μL, 3.59mmol), reacted at room temperature for 4-5h, observed by TLC. After the reaction, add saturated K 2 CO 3 The solution was quenched, the solvent was distilled off under reduced pressure, extracted with dichloromethane (3×10mL), washed once with water, washed 2-3 times with saturated brine, added anhydrous MgSO4 Dry, filter, combine the organic phases, and distill off the solvent under reduced pressure to obtain 680 mg of light yellow solid with a yield of 98.8%.
[0029] 1 H NMR (400MHz, CDCl 3 )δ8.08(t, J=9.2Hz, 1H), 7.88(t, J=7.8Hz, 1H), 7.70(d, J=7.7Hz, 1H), 4.65(s, 2H), 4.02(s, 3H).
Embodiment 3
[0030] The preparation of embodiment 3 compound e:
[0031] Compound d (2 mL, 16.45 mmol) was added into a round bottom flask containing dichloromethane (45 mL), and stirred evenly. Take another small beaker, add dichloromethane (5 mL) and ethylenediamine (659 μl, 9.87 mmol), stir and mix well. Then it was gradually dropped into a round bottom flask, refluxed at 90°C, and reacted overnight. After rotary evaporation, a yellow oily liquid was obtained, which was dissolved and cooled with ethyl acetate, and 2.21 g of a yellow-white solid e was obtained by suction filtration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com