Preparation method for carbon tetrachloride
A technology of carbon tetrachloride and carbon disulfide, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve problems such as improper handling of by-products, low purity of finished products, and low investment, so as to improve sufficiency and improve reaction Efficiency, the effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
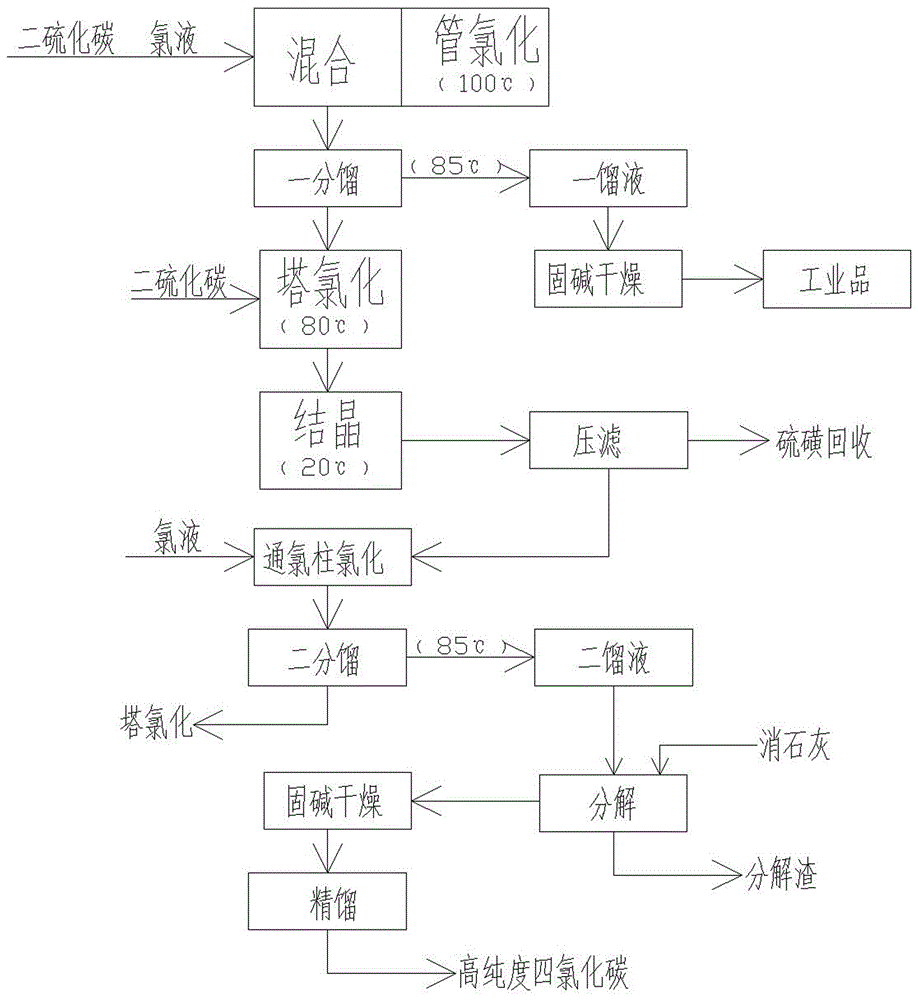
[0020] Such as figure 1 A kind of preparation method of carbon tetrachloride shown, comprises the following steps:
[0021] Step 1: Tube chlorination: Mix carbon disulfide with a purity of 98% and chlorine solution with a purity of 99% at a weight ratio of 1:2, and then add it to a coiled tube reactor for reaction. The coiled tube reactor is placed in water, and the water temperature is controlled at 100°C , the reaction vapor after the reaction is generated and introduced into the fractionation tower to carry out a fractionation;
[0022] Step 2: Fractionation tower one fractionation: Fractionation temperature is 85 ℃, collects a distillate carbon tetrachloride at the top of the fractionation tower, and a distillate is dried through a solid alkali drier to obtain low-purity carbon tetrachloride, a fractionation tower Store liquid disulfide dichloride for tower chlorination;
[0023] Step 3: tower chlorination: the fractionation tower is fully refluxed, and an excess of 25% ...
Embodiment 2
[0029] The difference between Example 2 and Example 1 is that the purity of carbon disulfide in step 1 is 97%, and the water temperature is 95°C; the fractionation temperature in step 2 is 83°C; the fractionation temperature in step 6 is 80°C.
Embodiment 3
[0031] The difference between embodiment 3 and embodiment 1 is that the water temperature in step one is 98°C; the fractionation temperature in step two is 80°C; the fractionation temperature in step six is 82°C.
[0032] Compared with the carbon disulfide chlorination method adopted in the background technology, the purity of the carbon tetrachloride prepared by the present invention is increased by 4 to 5 percentage points, and the by-products are fully utilized simultaneously, greatly reducing the pollution to the environment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com