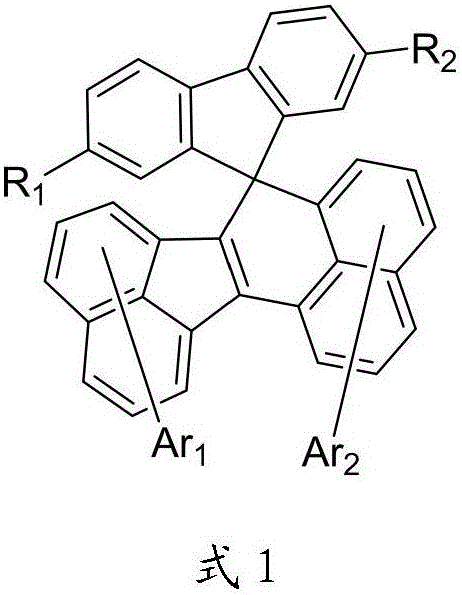
Organic photoelectric material containing spiral ring structure and application thereof
A technology of organic optoelectronic materials and spiro structure, which is applied in the fields of luminescent materials, organic chemistry, circuits, etc., can solve the problems of high driving voltage, low luminous brightness and efficiency, and has not attracted too much attention, and achieves improved quantum efficiency, Good application effect, power efficiency and quantum efficiency improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
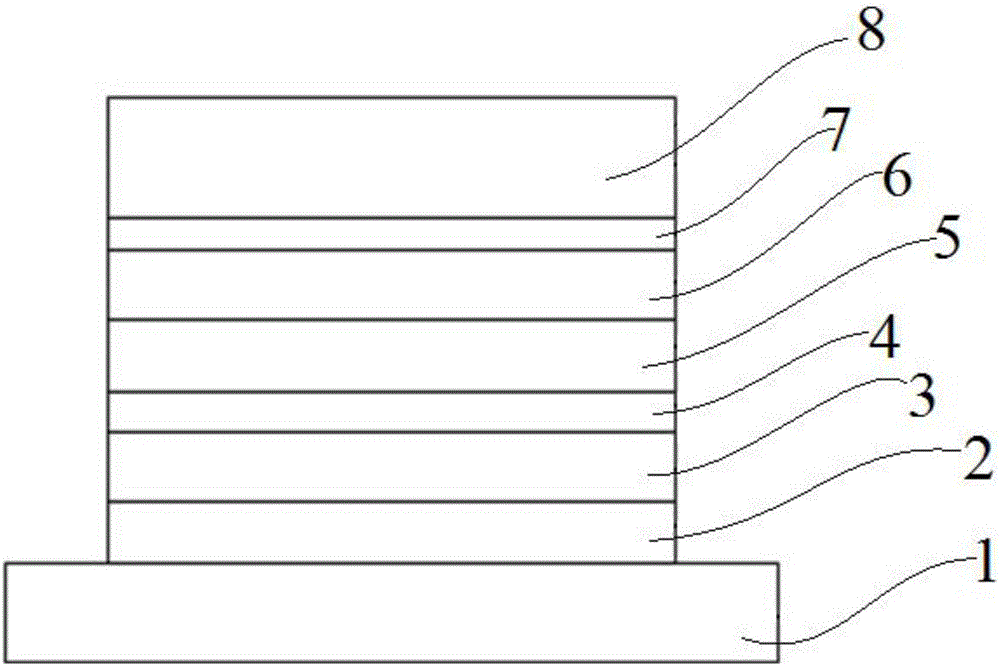
Image
Examples
Embodiment 1
[0082] Example 1 Preparation Example of Organic Optoelectronic Material
Embodiment 1
[0083] The preparation of embodiment 1 compound 1
[0084] (1) Preparation of compound 1-a:
[0085]
[0086] At 45-50°C and under the protection of nitrogen, take 21.6g 5-methoxy-1-naphthoic acid methyl ester (100.0mmol) and 17.8g (100mmol) NBS (N-bromosuccinimide) Disperse in 200g of dichloroethane, heat preservation reaction for 2.0 hours, then pour the above system into 200g of saturated sodium sulfite aqueous solution, stir and react for 30min, the system is layered, washed with water, and finally the organic phase is desolvated under reduced pressure to free Fraction, 29.5 g of compound 1-a was obtained without further purification.
[0087] (2) Preparation of compound 1-b:
[0088]
[0089] Get the 29.5g compound 1-a (100.0mmol), 20.7g potassium carbonate (150mmol), 80g water, 28.1g 6-chloro-1-acenaphthylenylboronic acid pinacol ester (90mmol) and 260g obtained in the step (1). Toluene, under the protection of nitrogen, add catalyst 0.9g Pd (PPh 3 ) 4 (0.75mm...
Embodiment 2
[0115] The preparation of embodiment 2 compound 4
[0116] (1) Preparation of compound 4-a:
[0117]
[0118] Take 28.6g 1,8-dibromonaphthalene (100.0mmol), 10.4g potassium carbonate (75mmol), 70g water, 15.6g 6-chloro-1-acenaphthyleneneboronic acid pinacol ester (50mmol) and 400g toluene, Under the protection of , add catalyst 0.9g Pd(PPh 3 ) 4 (0.75mmol), then heated to reflux, while adopting thin-layer chromatography (TLC) to track the reaction process, after about 18.0 hours of reaction, after being down to room temperature, the system was layered, and then after washing with water, the organic phase was decompressed. Solvent until there is no distillate, and then use petroleum ether ethyl acetate mixture to carry out column chromatography purification on the residue, the volume ratio of petroleum ether and ethyl acetate is petroleum ether:ethyl acetate=30:1, to obtain 8.4g compound 4-a , and the yield was 43.08%.
[0119] (2) Preparation of compound 4-b:
[0120] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com