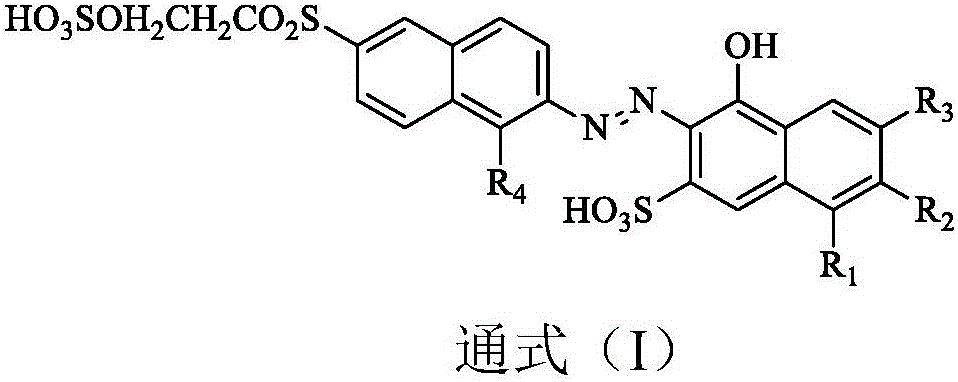
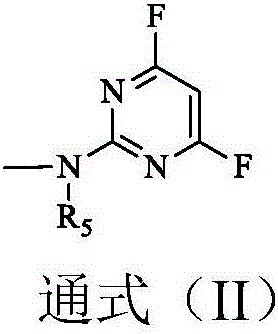
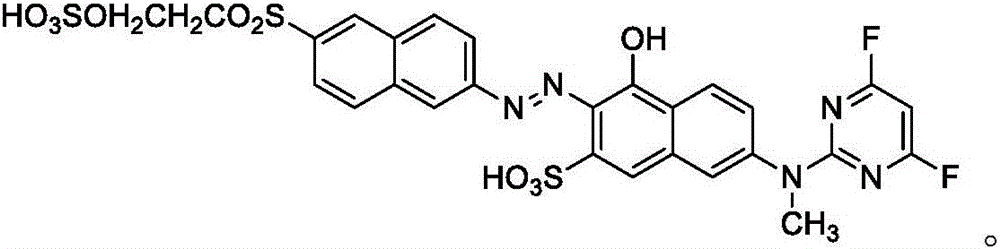
Reactive orange dye compound and preparation method thereof
A compound, orange dye technology, applied in the field of reactive orange dye compounds and their preparation, can solve the problems of increased dye fixation rate, unmatched activity of reactive groups, etc., and achieves the effect of excellent fastness performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolve 25.3 parts of N-methyl J acid in 200 parts of water, add 10% sodium carbonate solution to adjust the pH to 6, completely dissolve, then add 13.5 parts of trifluoropyrimidine at a temperature of 20°C and a pH of Under the condition of 7, carry out condensation 5h, when there is no N-methyl J acid, it is the end point, and the condensation product is obtained;
[0027] (2) Get 33.1 parts of the diazonium salt of 2-naphthylamine-6-(β-ethylsulfone sulfate), and the condensation product obtained in step (1) is 15 ℃ at a temperature, and the pH is 6 conditions, coupling After reacting for 5 h, Compound A was obtained; its molecular weight was 709 by HPLC-MS, which was consistent with its structure.
[0028]
Embodiment 2
[0030] (1) Dissolve 23.9 parts of J acid in 200 parts of water, add 10% sodium carbonate solution to adjust the pH to 6, completely dissolve, then add 13.5 parts of trifluoropyrimidine at 10°C, and condense under the condition of pH 9 Reaction 6h, when there is no J acid, it is the end point, and the condensation product is obtained;
[0031] (2) Get the diazonium salt of 41.1 parts of 2-naphthylamine-6-(β-ethylsulfone sulfate)-1-sulfonic acid, and the condensation product that step (1) makes, at 15 ℃, pH is Under the conditions of 7, the coupling reaction was carried out for 5 hours to obtain compound B; its molecular weight was 770 by HPLC-MS, which was consistent with its structure.
[0032]
Embodiment 3
[0034] Compound C was obtained by a method similar to that of Example 1; its molecular weight was 789 as measured by HPLC-MS, which was consistent with its structure.
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com