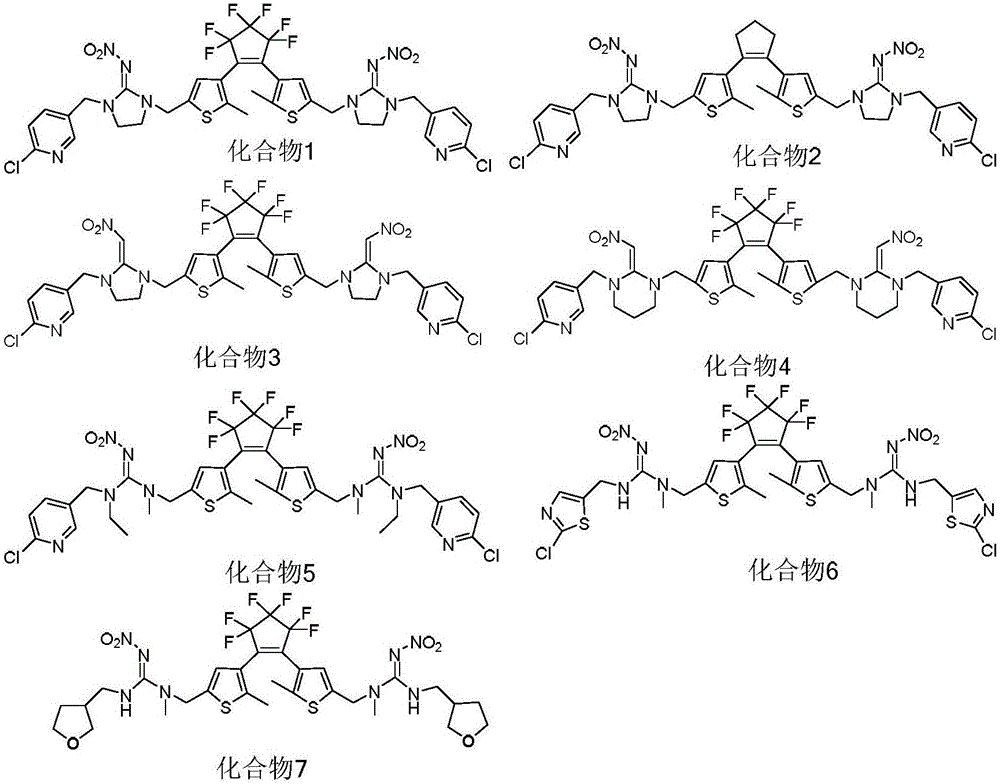
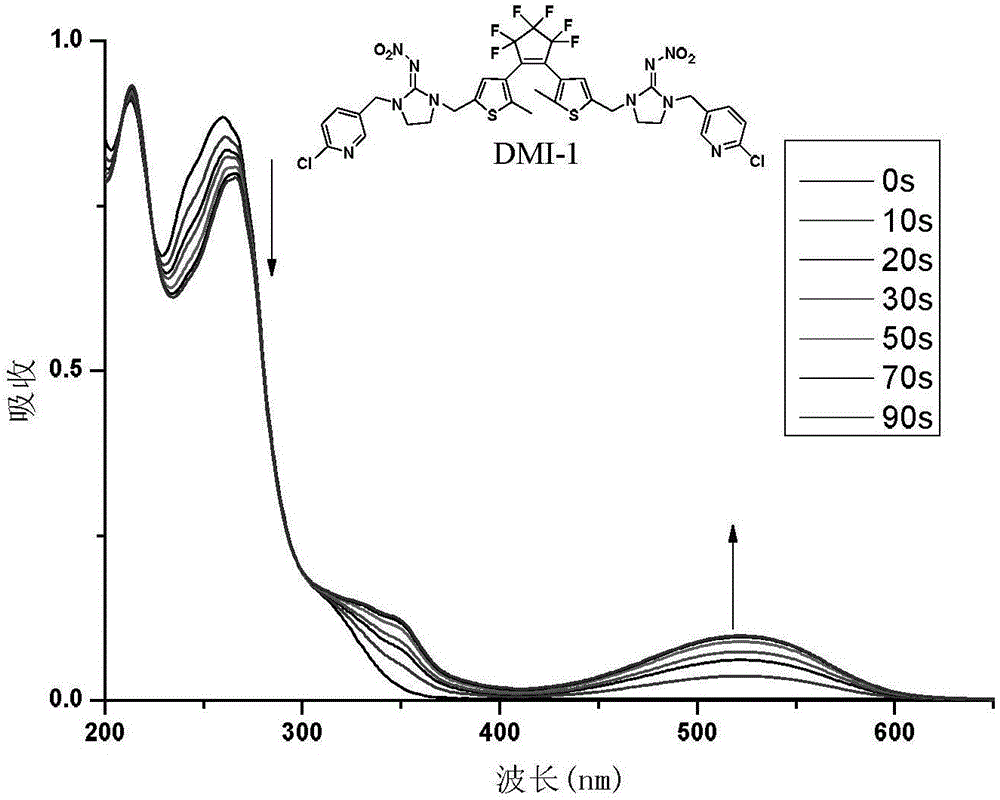
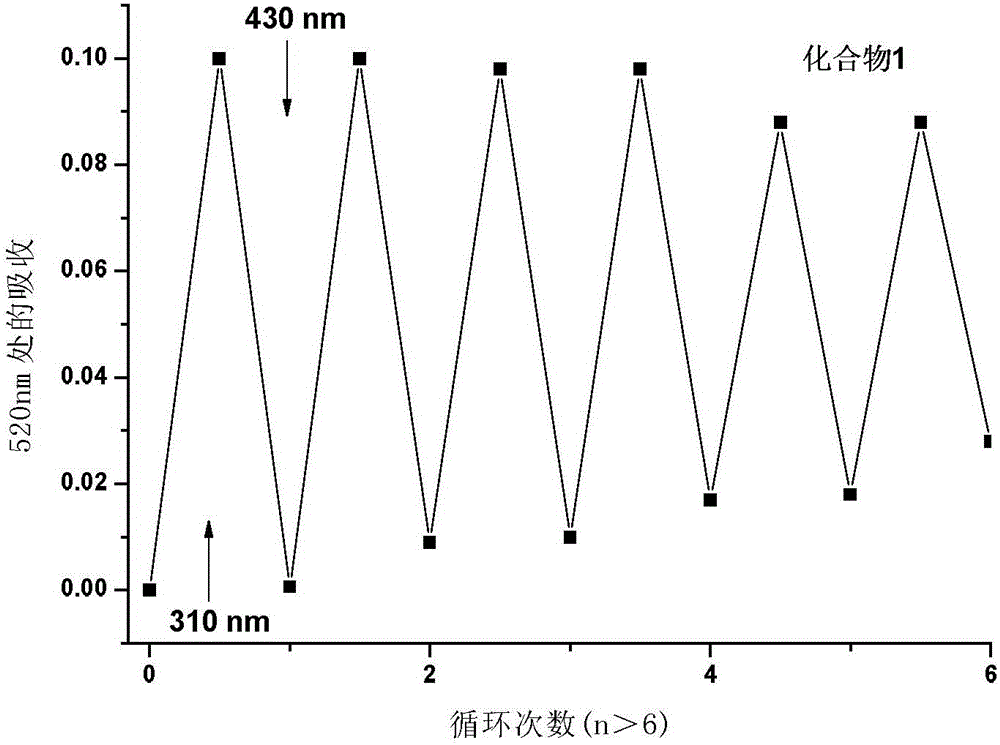
Diarylethene type photochromic insecticidal compound and preparation method and purpose thereof
A technology for compounds and aromatic ring groups, applied in the field of diarylethene photochromic compounds and their preparation, can solve the problems of limited drug selectivity, narrow insecticidal spectrum, restricted compound development and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0109] The preparation method of the compound of the present invention
[0110] The compound represented by the general formula (A) of the present invention can be prepared by the following method, but the conditions of the method, such as the amount of reactant, solvent, acid-base, compound used, reaction temperature, reaction time required, etc. are not limited to the following explanation of. The compounds of the present invention can also optionally be conveniently prepared by combining various synthetic methods described in this specification or known in the art, such combinations can be easily performed by those skilled in the art to which the present invention pertains.
[0111] The compound represented by general formula (A) of the present invention can be synthesized by following method (A), and described method comprises steps:
[0112] (1) in an inert solvent, the compound of formula (1a) is reacted with a brominating reagent (such as bromine), thereby forming a ...
Embodiment 1
[0148] The synthesis of embodiment 1 compound 1
[0149] 1.1 Preparation of 4-bromo-5-methyl-2-thiophenecarbaldehyde
[0150]
[0151]Add 5-methyl-2-thiophenecarbaldehyde (6.3g, 50.0mmol) and 50mL acetic acid into a 100mL round-bottomed flask, then weigh Br with a constant pressure dropping funnel 2 (8.78g, 55.0mmol) was dissolved in an appropriate amount of acetic acid, and the bromine acetic acid solution was slowly added dropwise at room temperature, and stirred at room temperature for 24 hours. After the reaction was followed by TLC, water was added to stop the reaction. The reaction mixture was neutralized to neutral with anhydrous sodium carbonate. The product was extracted three times with diethyl ether, the organic phase was dried with anhydrous sodium sulfate, filtered, and the solvent was removed under reduced pressure to obtain a black liquid, which was eluted with petroleum ether:ethyl acetate=40:3 (v:v) for column chromatography. The solvent was evaporated to...
Embodiment 2
[0170] The synthesis of embodiment 2 compound 2
[0171] 2.1 Preparation of 1,5-bis(5-chloro-2-methylthiophen-3-yl)pentane-1,5-dione
[0172]
[0173]Add anhydrous aluminum trichloride (12.1g, 90.49mmol) and 150mL of nitromethane successively in a 50mL round-bottomed flask. Under ice-bath conditions, first add a nitromethane solution of glutaryl chloride (6.37, 37.71mmol) dropwise, A solution of 2-chloro-5-methylthiophene (10 g, 75.41 mmol) in nitromethane was added dropwise. After the addition, continue to react for 0.5h, then remove the ice bath, continue to stir the reaction at room temperature for 6h, TLC tracking after the end of the reaction, slowly add 60mL of ice-water mixture carefully, continue stirring for 5min, separate the organic phase, the water layer Extract with anhydrous ether (50mL×2), combine the organic phases, adjust to neutral with saturated sodium bicarbonate solution, then wash with water, wash with saturated sodium chloride solution, dry over anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com