An ionic liquid covalently modified polyacid intercalated hydrotalcite and its application in deep desulfurization of oil products
An ionic liquid, covalent modification technology, applied in the petroleum industry, refining hydrocarbon oil, processing hydrocarbon oil, etc., to achieve the effect of simple preparation method, improved thermal stability and recovery rate, and promotion of contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
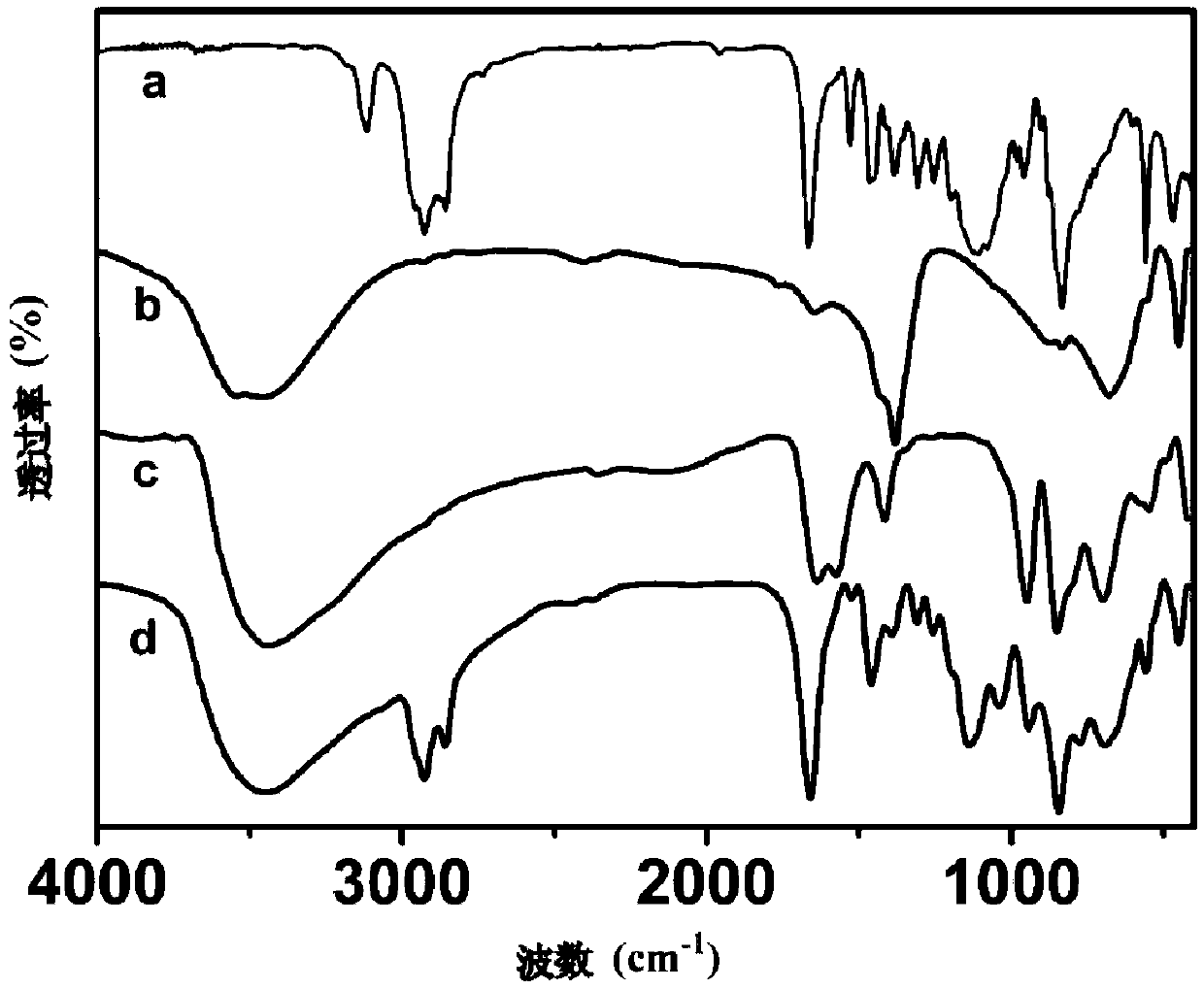
Image
Examples
Embodiment 1
[0021] a. Take 0.03mol Mg(NO 3 ) 2 ·6H 2 O, 0.01mol Mg(NO 3 ) 2 ·6H 2 O mixed, dissolved in 80mL deionized water, and then 0.01mol hexamethylenetetramine was added to the above mixed salt solution, centrifuged at 3600rpm / min for 10min, the supernatant was transferred to an autoclave, and hydrothermally heated at 140°C Crystallize for 24h, then wash with suction to obtain a hydrotalcite precursor [Mg 3 Al(OH) 8 ]CO 3 .
[0022] b. Take 1g of [Mg 3 Al(OH) 8 ]CO 3 Disperse in 100mL methanol solution, pass through inert protective gas and stir for 3-5h, add 0.5mL of concentrated nitric acid into the dispersion system, stir for 3-5h, wash to neutral after the reaction, and obtain water with nitrate as the interlayer anion Talc precursor [Mg 3 Al(OH) 8 ]NO 3 .
[0023] c. Take 0.1g of [Mg 3 Al(OH) 8 ]NO 3 , stirred in 100mL formamide solvent for 24h under the condition of inert protective gas, centrifuged the hydrotalcite solution after peeling off, and discarded t...
Embodiment 1
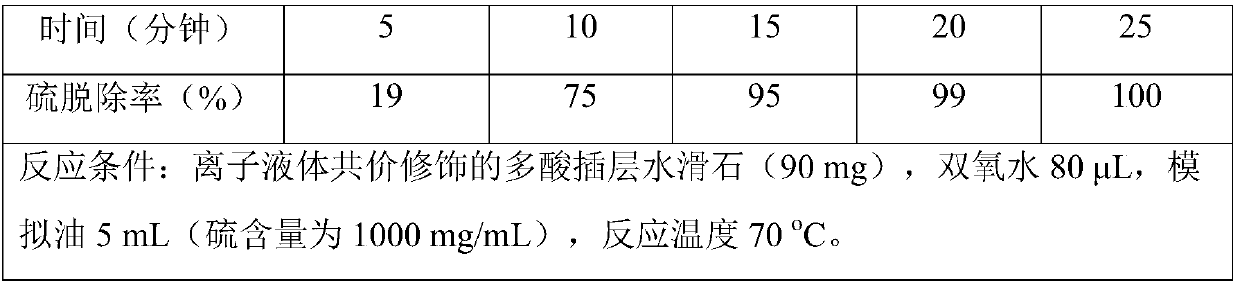
[0026] The catalytic reaction is carried out in a simple glass reactor with magnetic stirring. Weigh 90mg of the multi-acid intercalated hydrotalcite covalently modified by the ionic liquid prepared in Example 1 as a catalyst, then add 5mL of simulated oil (wherein the sulfur concentration of the desulfurization substrate is 1000ppm) and 0.080mL of 30wt% hydrogen peroxide, in React at 70°C, take samples from the reaction system at intervals of 5 minutes, analyze by gas chromatography, and calculate the removal rate of sulfur-containing substrates.
[0027] The catalytic results are shown in Table 1. It can be seen from Table 1 that the multi-acid intercalated hydrotalcite covalently modified by the ionic liquid prepared in Example 1 can convert 99.9% of 4,6-dimethyldiphenyl The thiophene is oxidized to form 4,6-dimethyldibenzothiophene sulfone, so as to achieve a significant removal effect.
[0028] Table 1:
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com