Macrolide benzoate compound, intermediate compound thereof, preparation method of the intermediate compound, and application of the macrolide benzoate compound
A technology of benzoate compounds and macrolides, applied in the field of pesticides, can solve the problems of low activity, utilization, waste and the like, and achieve the effects of high activity, simple preparation method and wide application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
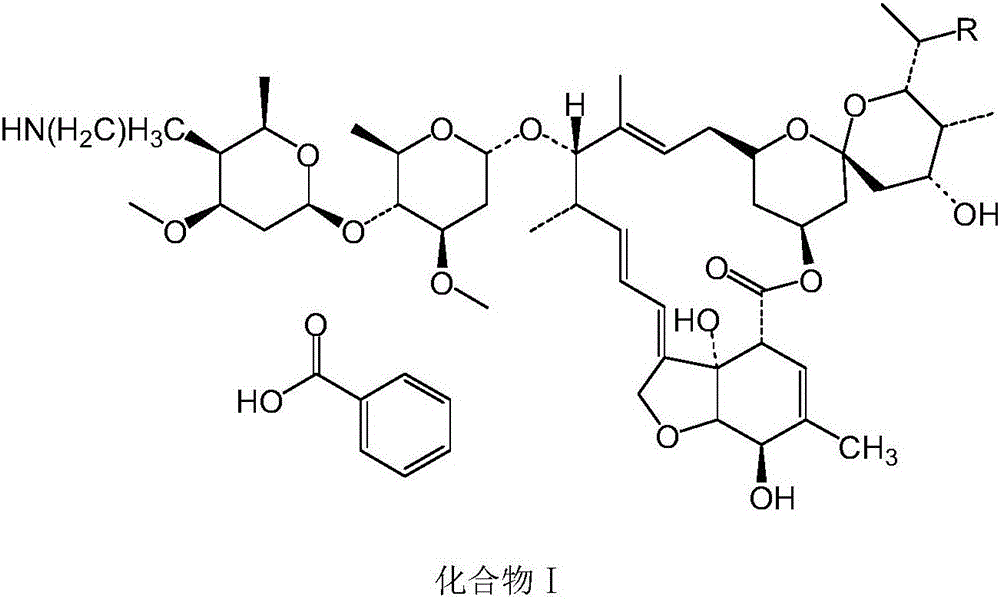
[0043] Embodiment 1: When R is methyl, the preparation of compound II
[0044] (1) 5-position hydroxyl protection: take compound a: Abamectin B 2b Dissolve in dichloroethane solvent, and cool the material liquid to -10~-25°C, then keep it warm for 1-2h. After the heat preservation is over, add the prepared allyl chloroformate and tetramethylethylenediamine solution dropwise at the same time to react; after the reaction is terminated, compound b: 5-protected avermectin B 2b Compound, compound a: allyl chloroformate: the molar ratio of tetramethylethylenediamine is 1:3~4:1~5;
[0045] (2) Oxidation of hydroxyl group at the 4″ position: Add compound b obtained in step (1), and then add tetramethylethylenediamine and dimethyl sulfoxide to mix well, and cool down to -13~-16°C, then dropwise add Phenyl phosphate dichloride, and keep the liquid temperature at -18°C for reaction; after the reaction is terminated, compound c is obtained after extraction: 5-O-allyl-4"-carbonyl-abamect...
Embodiment 2
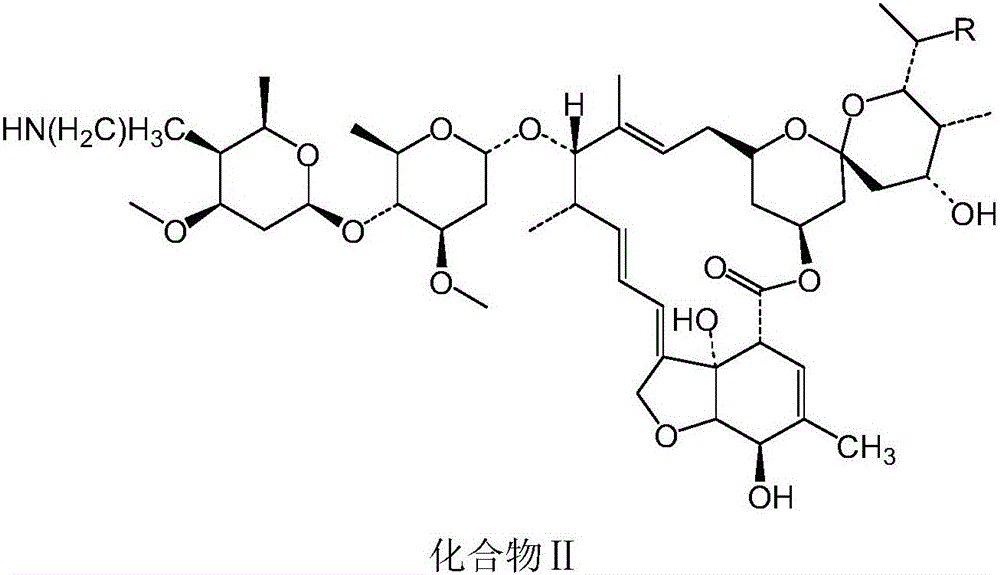
[0049] Embodiment 2: When R is ethyl, the preparation of compound II
[0050] (1) 5-position hydroxyl protection: take the avermectin B shown in formula a 2a Dissolve in dichloroethane solvent, and cool the feed solution to -18°C, then keep it warm for 1.5h. After the heat preservation is over, add the prepared allyl chloroformate and the ethane solution of tetramethylethylenediamine dropwise at the same time to react; after the reaction is terminated, the abamectin B with the 5-position protection shown in formula b is obtained 2a compound;
[0051] (2) Oxidation of hydroxyl group at the 4″ position: Add compound b obtained in step (1), and then add tetramethylethylenediamine and dimethyl sulfoxide to mix well, and cool down to -13~-16°C, then dropwise add Phenyl phosphate dichloride, and keep the liquid temperature at -18°C for reaction; after the reaction is terminated, the 5-O-formyl-4"-carbonyl-abamectin shown in formula c is obtained after extraction B 2a ;
[0052]...
experiment example 3
[0055] Experimental Example 3: Preparation of Compound I
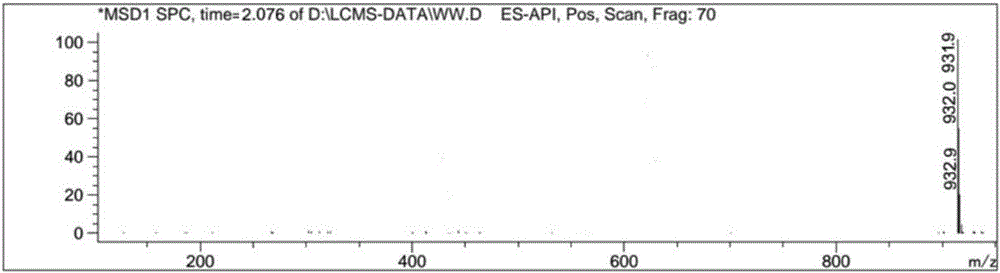
[0056] Take compound II prepared in Example 1 or 2, add benzoic acid with a molar ratio of 1:1.05 to compound II, and conduct a salt-forming reaction at a temperature of 75°C and a pressure of -0.09Mpa to obtain compound I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com