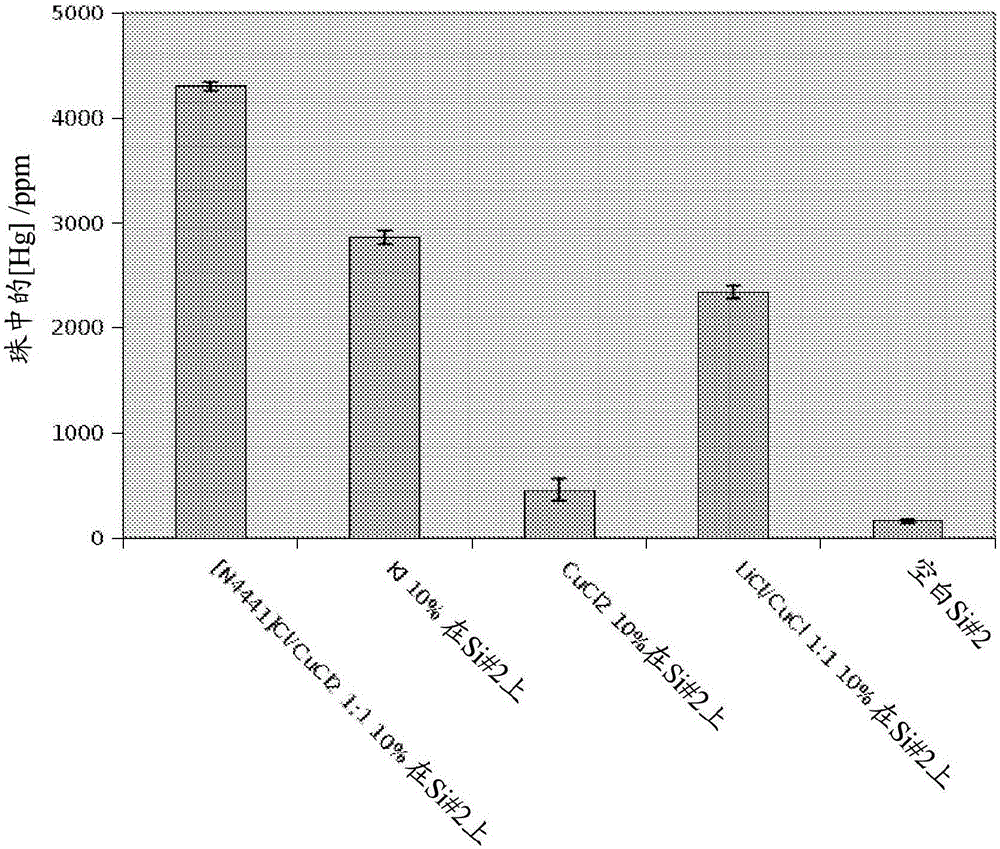
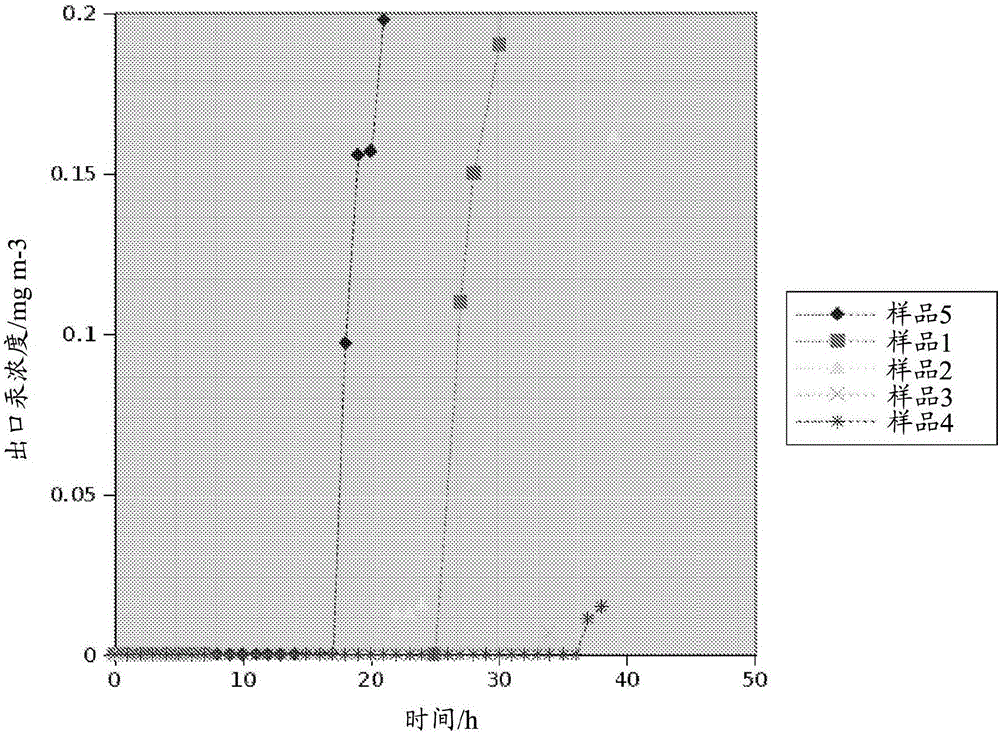
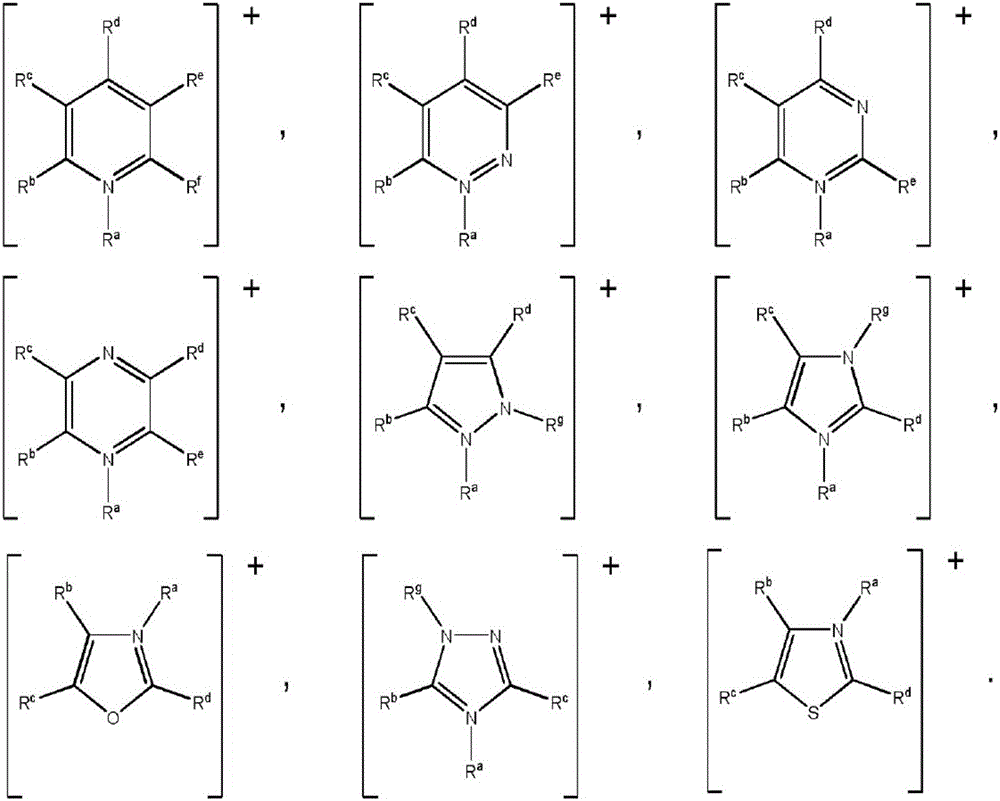
Process for removing metals from hydrocarbons
A technology, a technology of metal salts, applied in the direction of refining with metal salts, purification/separation of hydrocarbons, refining hydrocarbon oils, etc., can solve problems such as incompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0352] Example 1: Extraction of elemental mercury by an ionic liquid containing copper
[0353]1-Butyl-3-methylimidazolium chloride (5 g, 29 mmol) and copper(II) chloride dihydrate (5 g, 29 mmol) were combined in a flask and heated to 70 °C under vacuum to produce a tan color Viscous oil.
[0354] 0.037 g of the above oil was contacted with 0.0183 g of elemental mercury and heated to 60° C. overnight in a sealed tube to produce a pale, slightly blue ionic liquid containing an off-white precipitate. will be 10cm 3 Deionized water was added to the mixture, filtered, and diluted to 50cm 3 . Dilute 1 cm with deionized water 3 The dilute solution is further diluted to 50cm 3 . The resulting solution was analyzed for mercury using a Milestone DMA-80 direct mercury analyzer. The solution was found to contain 3.27 ± 0.21 ppm mercury, indicating that 0.22 g mercury per gram of initial ionic liquid had been converted to a water-soluble ionic form, in contrast to the theory of 0...
Embodiment 2
[0355] Example 2: Extraction of Elemental Mercury by Copper-Containing Ionic Liquids
[0356] The copper(II) containing ionic liquid of Example 1 was contacted with 0.355 g of elemental mercury and heated to 60° C. overnight in a sealed tube. The resulting mixture was analyzed as in Example 1 and found to contain 7.02 ± 0.24 ppm mercury in the analyte solution, corresponding to 18.6 wt% dissolved mercury.
Embodiment 3
[0357] Example 3: Extraction of elemental mercury from dodecane
[0358] Ionic liquids were prepared from a 2:1 molar ratio of 1-butyl-3-methylimidazolium chloride and copper(II) chloride dihydrate. 0.18 g of ionic liquid was added to a 10 cm 3 A sample vial of dodecane was added, and the resulting mixture was stirred overnight at 60 °C. The resulting dodecane phase was then analyzed for total mercury concentration and found to contain 34.9 ppb mercury.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com