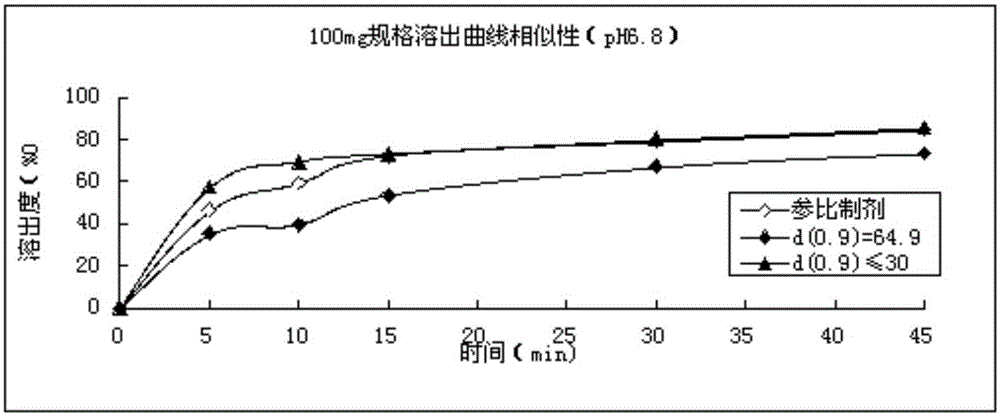
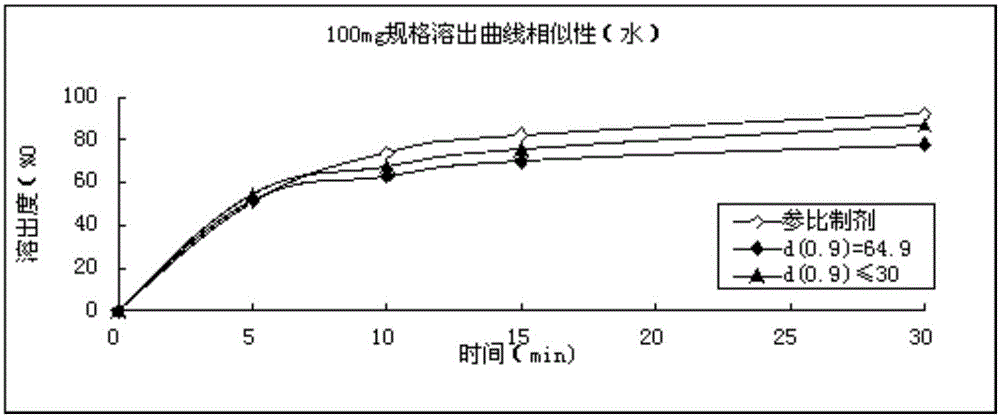
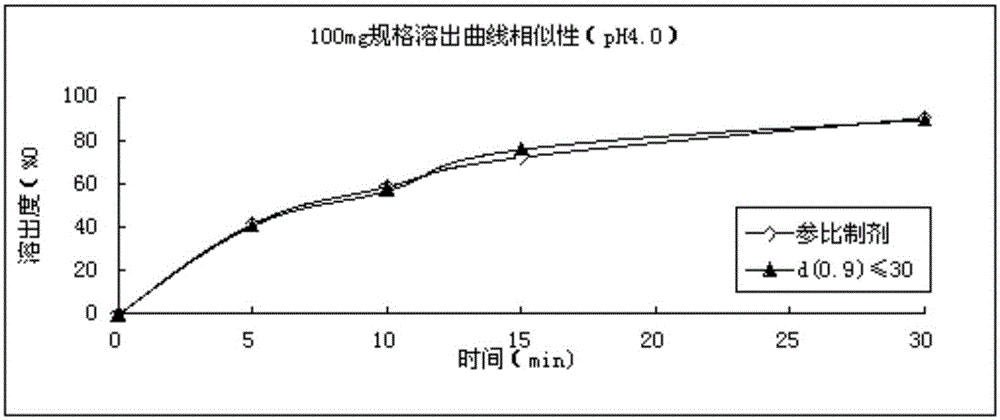
Refining method for avanafil
A technology of avanafil and refining method, which is applied in the field of medicine, can solve the problems of poor dissolution effect and insufficient dissolution rate, and achieve the effects of high purity and yield, easy operation and good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] At room temperature, 20 g of crude avanafil with a purity of 98.5% (as determined by HPLC) was added to a mixed solvent of 280 mL of ethyl acetate and 140 mL of absolute ethanol, stirred evenly, heated to reflux, and after the solid was completely dissolved, let it stand naturally After cooling down to 40°C, start stirring, and cool down to 10°C in an ice bath. Incubate for 1 hour, filter, collect the filter cake, and dry under vacuum (-0.09Mpa) at 40-50°C for 8 hours to obtain 16 g of avanafil with a purity of 99.91% (as determined by HPLC). Yield: 80%.
[0032] Solvent residue: ethyl acetate, 0.08%, ethanol 0.10% (as determined by GC), particle size: ≤30 μm.
Embodiment 2
[0034] At room temperature, 50 g of avanafil crude product with a purity of 98.5% (as determined by HPLC) was added to a mixed solvent of 300 mL of methanol and 600 mL of isopropyl ether, after stirring evenly, it was heated to reflux, and after the solid was completely dissolved, it was allowed to stand and naturally cooled to 30°C, start stirring, cool down to 0°C in an ice bath, keep warm for 3 hours, filter, collect the filter cake, and dry under vacuum (-0.09Mpa) at 40-50°C for 8 hours to obtain 41g of avanafil with a purity of 99.90% (as determined by HPLC ). Yield: 82%.
[0035] Solvent residue: no detection of isopropyl ether, 0.13% methanol (measured by GC), particle size: ≤30 μm.
Embodiment 3
[0037] At room temperature, add 100 g of crude avanafil with a purity of 98.5% (as determined by HPLC) into a mixed solvent of 100 mL of dimethyl sulfoxide and 500 mL of ethyl acetate, stir and heat to reflux. After the solid is completely dissolved, cool down naturally To 20°C, start stirring, slowly cool down to -10°C in an ice bath, keep warm for 2 hours, filter, collect filter cake, and dry under vacuum (-0.09Mpa) at 75-80°C for 12 hours to obtain 83.5g of avanafil with a purity of 99.93% (HPLC determination). Yield: 83.5%.
[0038] Solvent residues: 0.15% dimethyl sulfoxide, 0.19% ethyl acetate (measured by GC), particle size: ≤30 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com