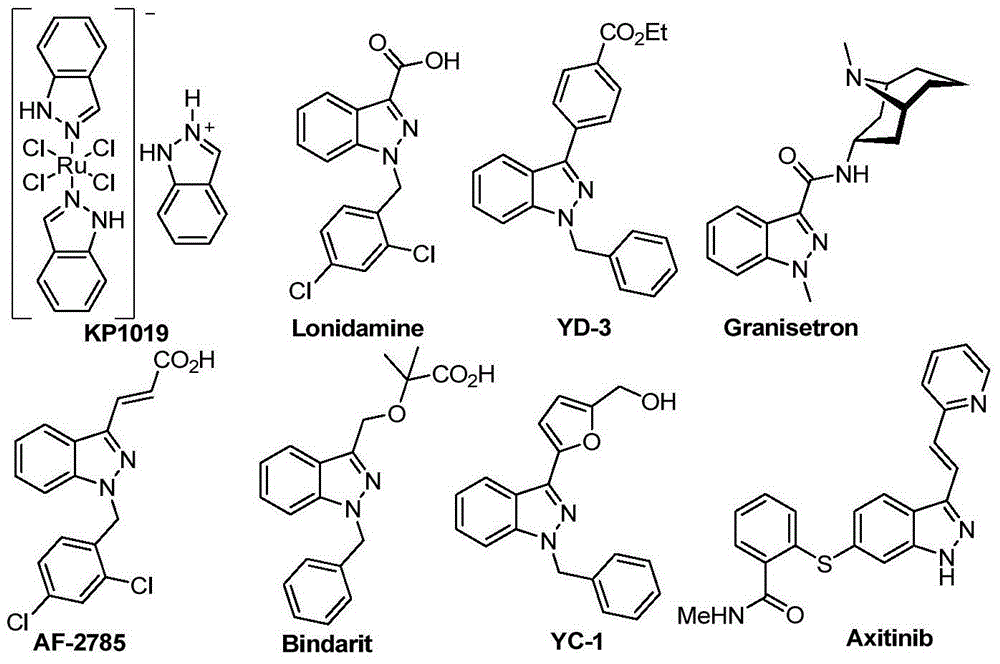
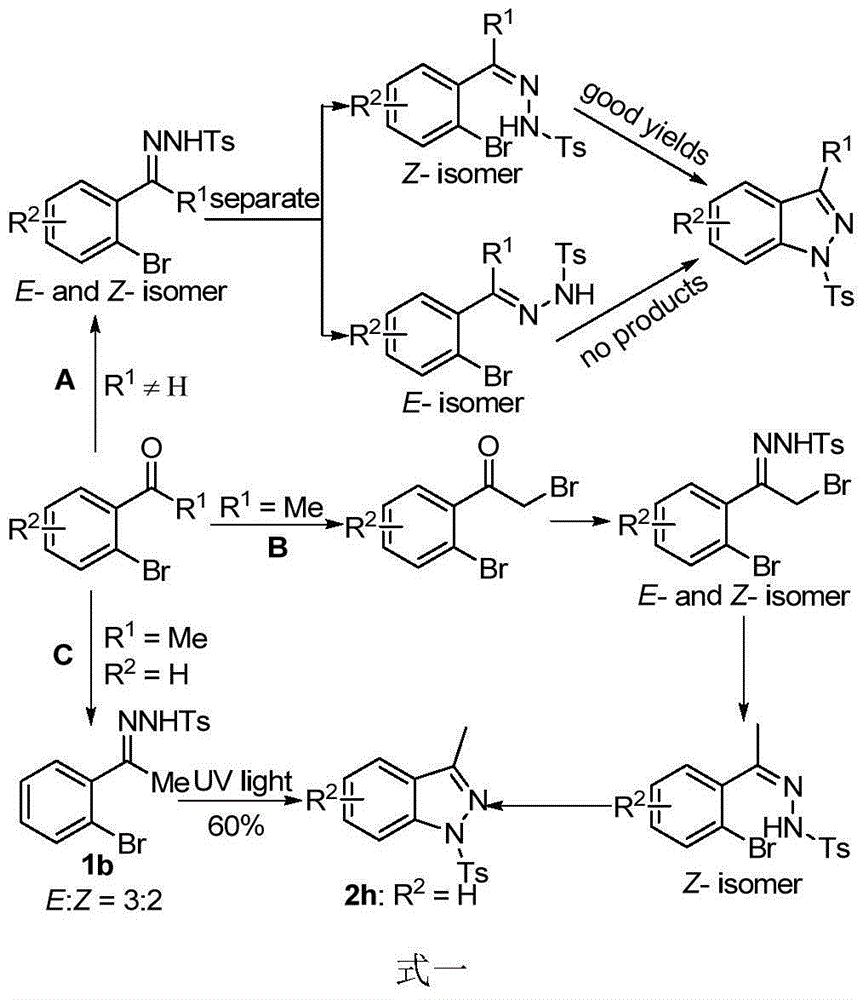
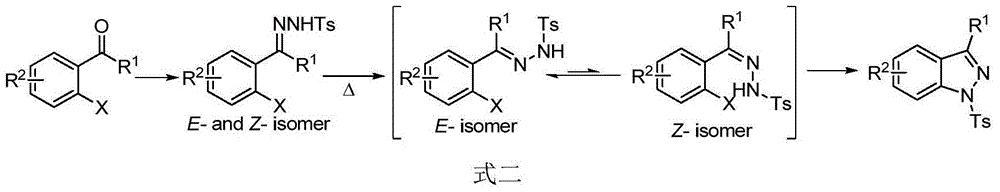
Preparation method of indazole and application of indazole in medicine synthesis
A technology of indazole and reaction, applied in the field of chemistry, can solve problems such as no product obtained, tolerance of unfavorable substrate functional groups, narrow scope of application of substrates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the preparation of indazole compound 2:
[0034]
[0035] Under argon protection, p-toluenesulfonylhydrazone 1 (0.2mmol) was dissolved in i-AmOH (2mL), and Cu 2 O (0.1mmol), heated and refluxed until p-toluenesulfonylhydrazone 1 disappeared completely (1-10 hours), the product was extracted with ethyl acetate (200mL), the organic phase was washed with salt water, and anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and the indazole compound 2 was obtained by column chromatography.
[0036] The spectrogram data is as follows:
[0037]
[0038] 1 H NMR (400MHz, CDCl 3 )δ=2.35(s,3H),7.23(d,J=8.4Hz,2H),7.30-7.34(m,1H),7.53-7.58(m,1H),7.68(d,J=8.0Hz,1H ), 7.87 (d, J = 8.4Hz, 2H), 8.18-8.23 (m, 2H) ppm; 13 C NMR (100MHz, CDCl 3 )δ=21.6, 113.1, 121.3, 124.1, 125.8, 127.5, 129.2, 129.8, 134.6, 140.3, 141.2, 145.3ppm; HRMS (ESI): Calcd for C 14 h 13 N 2 o 2 S[M+H] + :273.0692,found:273.0695.
[0039]
[0040] 1 H...
Embodiment 2 1
[0065] Embodiment two, the preparation of 1H-indazole 3:
[0066]
[0067] Route 1: Under argon protection, dissolve compound 2 (0.2 mmol) in 2 mL of methanol, add magnesium powder, react at room temperature for 3 hours, evaporate methanol under reduced pressure, extract with ethyl acetate, and use saturated ammonium chloride solution and Washed with saline, anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and compound 3 was obtained by column chromatography.
[0068]
[0069] 1 H NMR (400MHz, CDCl 3 )δ=7.16-7.20(m,1H),7.36-7.42(m,1H),7.52(dd,J=8.4,0.8Hz,1H),7.78(d,J=8.4Hz,1H),8.13(s ,1H),10.0(brs,1H)ppm; 13 C NMR (100MHz, CDCl 3 )δ=109.8, 120.9, 121.0, 123.1, 126.9, 134.7, 140.0ppm.
[0070]
[0071] 1 H NMR (400MHz, CDCl 3 )δ=3.86(s,3H),7.09-7.11(m,2H),7.40(d,J=9.6Hz,1H),8.03(brs,1H)ppm.
[0072]
[0073] 1 H NMR (400MHz, CDCl 3 )δ=6.93-6.98(m,1H),7.16(d,J=9.2Hz,1H),7.71(dd,J=8.8,5.2Hz,1H),8.10(s,1H),10.35(brs,1H ) ppm...
Embodiment 3 1
[0086] Embodiment three, the synthesis of 1H-indazole-3-carboxylic acid:
[0087]
[0088] Under the protection of argon, dissolve o-bromoacetophenone (148mg, 0.74mmol) in methanol (5mL), add KOH (230mg, 4.09mmol) and iodobenzene diacetate (287mg, 0.89mmol) successively, and react at room temperature for 5 hours , methanol was distilled off under reduced pressure, extracted with ethyl acetate, the organic phase was washed with water and brine, anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure. The crude product was dissolved in methanol (5 mL), added 2M hydrochloric acid (2 mL), and reacted at room temperature for 12 hours. The methanol was distilled off under reduced pressure, extracted with ethyl acetate, and the organic phase was washed with saturated Na 2 CO 3 solution and brine wash, anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and compound 4 (121 mg, 76% yield) was isolated by column chromatography...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com