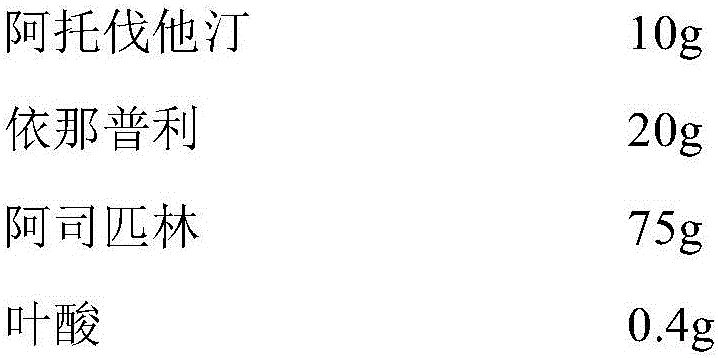Pharmaceutical composition for preventing cardiovascular diseases
A composition and drug technology, applied in the field of pharmacy, can solve the problem of weak cyclooxygenase effect, etc., and achieve the effects of preventing and treating atherosclerosis and inhibiting platelet aggregation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of compound atorvastatin / propranolol / aspirin / folic acid tablets
[0060] The prescription is as follows (1000 tablets):
[0061] Atorvastatin 10g
[0062] Propranolol 10g
[0063] Aspirin 75g
[0064] Folic acid 0.4g
[0065] Sodium Carboxymethyl Starch 20g
[0066] Calcium hydrogen phosphate 170g
[0067] 10% povidone aqueous solution appropriate amount
[0069] Preparation method: pulverize the raw and auxiliary materials through an 80-mesh sieve, and dry for use. Take 10 g of atorvastatin, 10 g of propranolol, 75 g of aspirin and 0.4 g of folic acid and mix them uniformly according to the equal increment method, add 20 g of sodium carboxymethyl starch and 170 g of calcium hydrogen phosphate, and mix them evenly according to the equal increment method, and the folic acid Dissolve in 10% povidone aqueous solution of binder, add an appropriate amount of binder to make soft material, granulate with 30 mesh, dr...
Embodiment 2
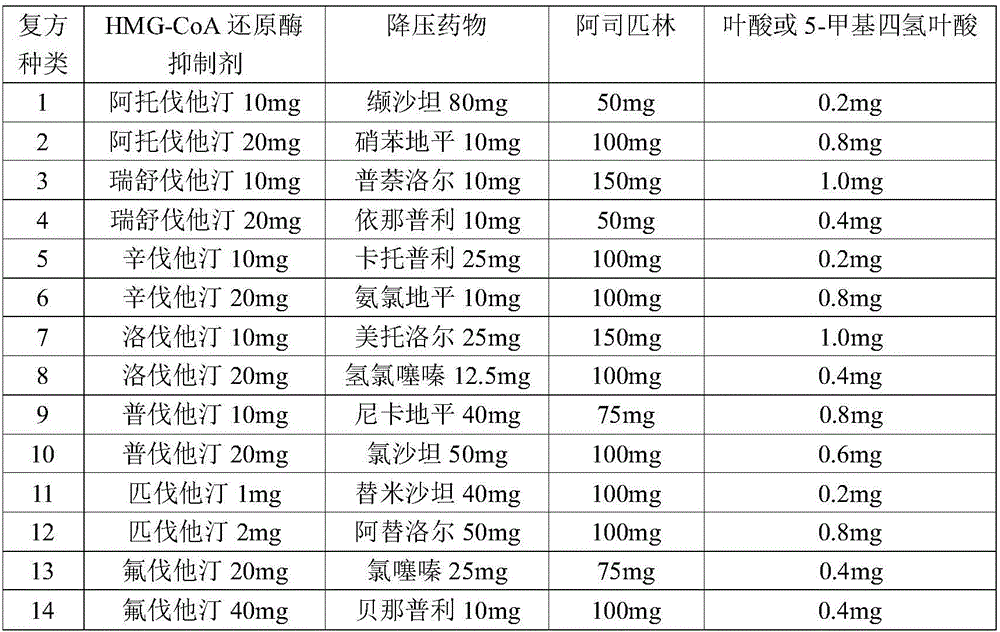
[0070] Example 2: Preparation of compound tablets
[0071] Preparation method: same as Example 1.
[0072] The components and contents of the 14 kinds of compound recipes are shown in Table 11.
[0073] Table 1 Composition of 15 kinds of active compound medicines
[0074]
Embodiment 3
[0075] Example 3: Preparation of compound atorvastatin / enalapril / aspirin / folic acid capsules
[0076] The prescription is as follows (1000 capsules):
[0077]
[0078]
[0079] Preparation method: pulverize the raw and auxiliary materials through an 80-mesh sieve, and dry for use. Take 10g of atorvastatin, 20g of enalapril, 75g of aspirin and 0.4g of folic acid and mix them uniformly according to the equal increment method. ), uniformly mix according to the equal increment method, make soft material with 10% povidone ethanol solution, granulate with 20 mesh sieve, dry at 60°C for about 2 hours, granulate with 20 mesh sieve, and control the water content of the granules to be 2-3 %, the dried granules are uniformly mixed with the prescription amount of magnesium stearate, the semi-finished product is tested, the content is measured, and then packed into hollow capsules to obtain 1000 capsules. Avoid light during preparation. After the finished product has passed the in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com