Monoclonal antibody for treating triple-negative breast cancer
A triple-negative breast cancer, monoclonal antibody technology, applied in the direction of antibodies, anti-tumor drugs, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of inability to develop drugs and lack of targeted therapy methods And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
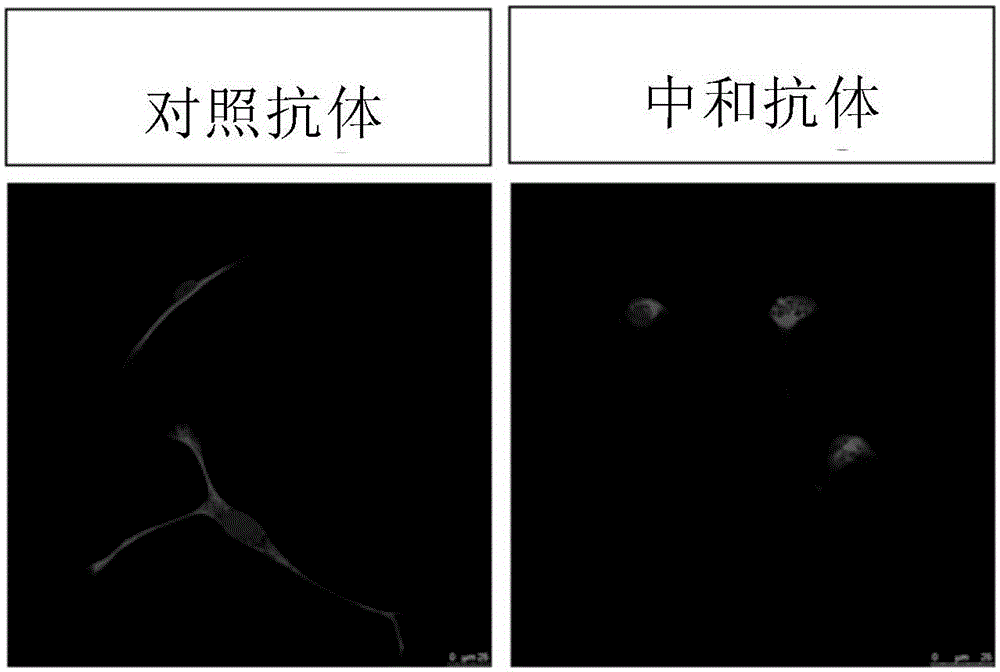
[0116] Example 1 Neutralizing antibodies can change the cell morphology of human triple-negative breast cancer cells
[0117] The human triple-negative breast cancer cell MDA-MB-231 (obtained from ATCC with high expression of PROCR. Product No. HTB-26 TM . ) were grown on coverslips at a relatively sparse density, and at the same time, 25ug / ml of neutralizing antibodies against PROCR (monoclonal antibody RCR-252, purchased from Abcam, catalog number ab81712) and anti-PROCR were added to the cell culture medium respectively. The control antibody of PROCR (purchased from Abcam, product number is ab56689), and Vim antibody (purchased from Cell Signaling Technology, product number is 5741P) was used for immunofluorescent staining after 12 hours.
[0118] The result is as figure 1 shown. The cell morphology of the MDA-MB-231 cells added with the control antibody did not change significantly, and was still long spindle-shaped, while the cell morphology of the cells added with t...
Embodiment 2
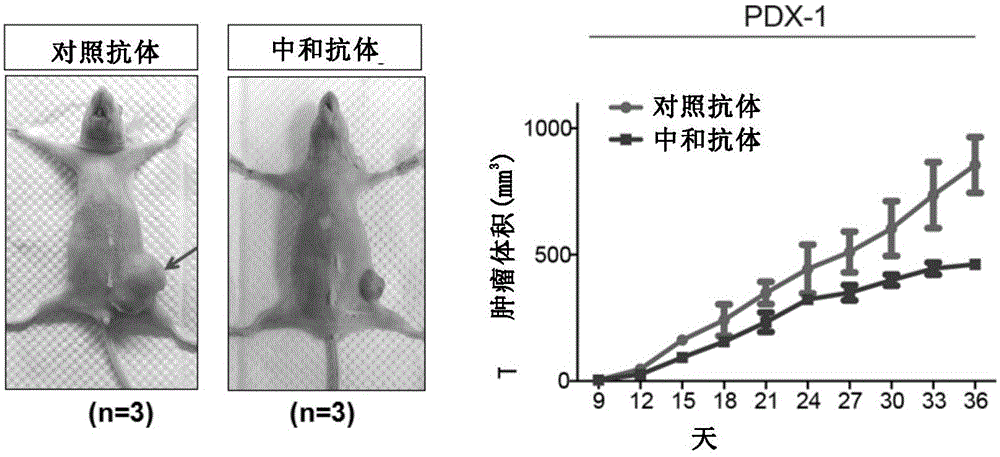
[0120] Example 2 Neutralizing antibodies inhibit the tumorigenicity of patient-derived triple-negative breast cancer (PDX) cells
[0121] Take female nude mice about 5 weeks old, and transfer patient-derived triple-negative breast cancer (PDX) cells to the same amount of cells (10 6 / only) were injected into the fourth pair of mammary glands on the right side of the mice. Nine days later, tumors began to form, and the mice were injected with control antibody and neutralizing antibody (monoclonal antibody RCR-252, purchased from Abcam, Cat. No. ab81712). The specific injection method is: intraperitoneal injection, the antibody dosage is 100ug / time. Inject every 2 days for a total of 3 injections per mouse. During this period, the size of the formed tumor was counted every 1 to 2 days.
[0122] The result is as figure 2 shown. The results showed that intraperitoneal injection of neutralizing antibodies was able to significantly inhibit the growth of patient-derived triple-...
Embodiment 3
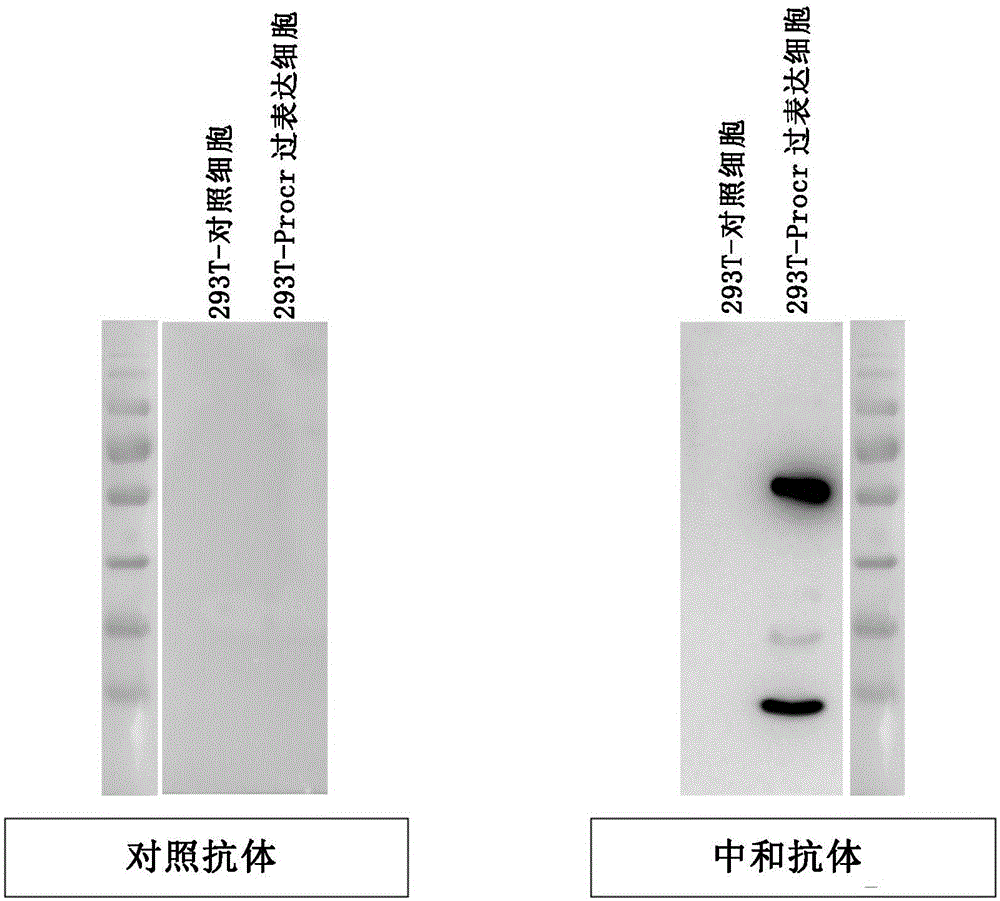
[0123] Example 3 Comparison of the ability of the control antibody and the neutralizing antibody (RCR-252) to bind to the PROCR protein
[0124] 3.1 Western blot
[0125] experimental method:
[0126] Human 293T cells were transfected with a human PROCR overexpression plasmid (the overexpression fragment is the full-length PROCR protein, and the overexpression plasmid was purchased from addgene, plasma No. 18121) by transient transfection of PEI. After 48 hours, the untransfected 293T cells of the control group and the 293T cells overexpressing PROCR were harvested. After lysing with protein lysate, centrifuge at 12000rpm / min for 10 minutes to remove cell debris, add 0.1M DTT and protein loading buffer, and boil at 100°C for 10 minutes. Store protein frozen at -80°C for later use.
[0127] The protein samples obtained from the 293T cells of the control group and the 293T cells overexpressing the PROCR protein in the experimental group were subjected to SDS-PAGE gel electrop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com