Nebulization of monoclonal antibodies for treating pulmonary diseases
a monoclonal antibody and pulmonary disease technology, applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve the problem that the systemic effect of the therapeutics cannot be observed using these methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0114] The following examples are offered by way of illustration and not by way of limitation.
Materials and Methods
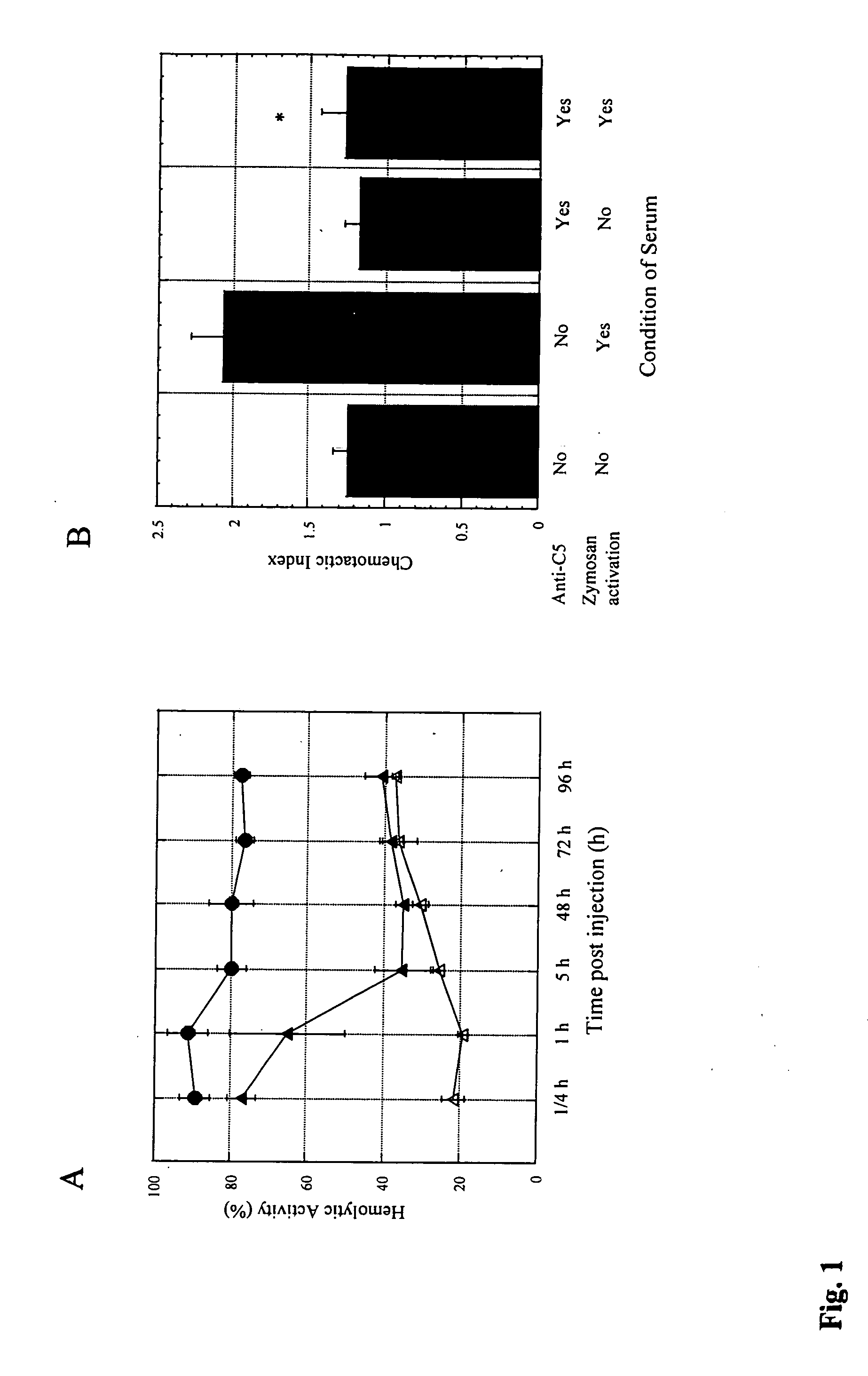
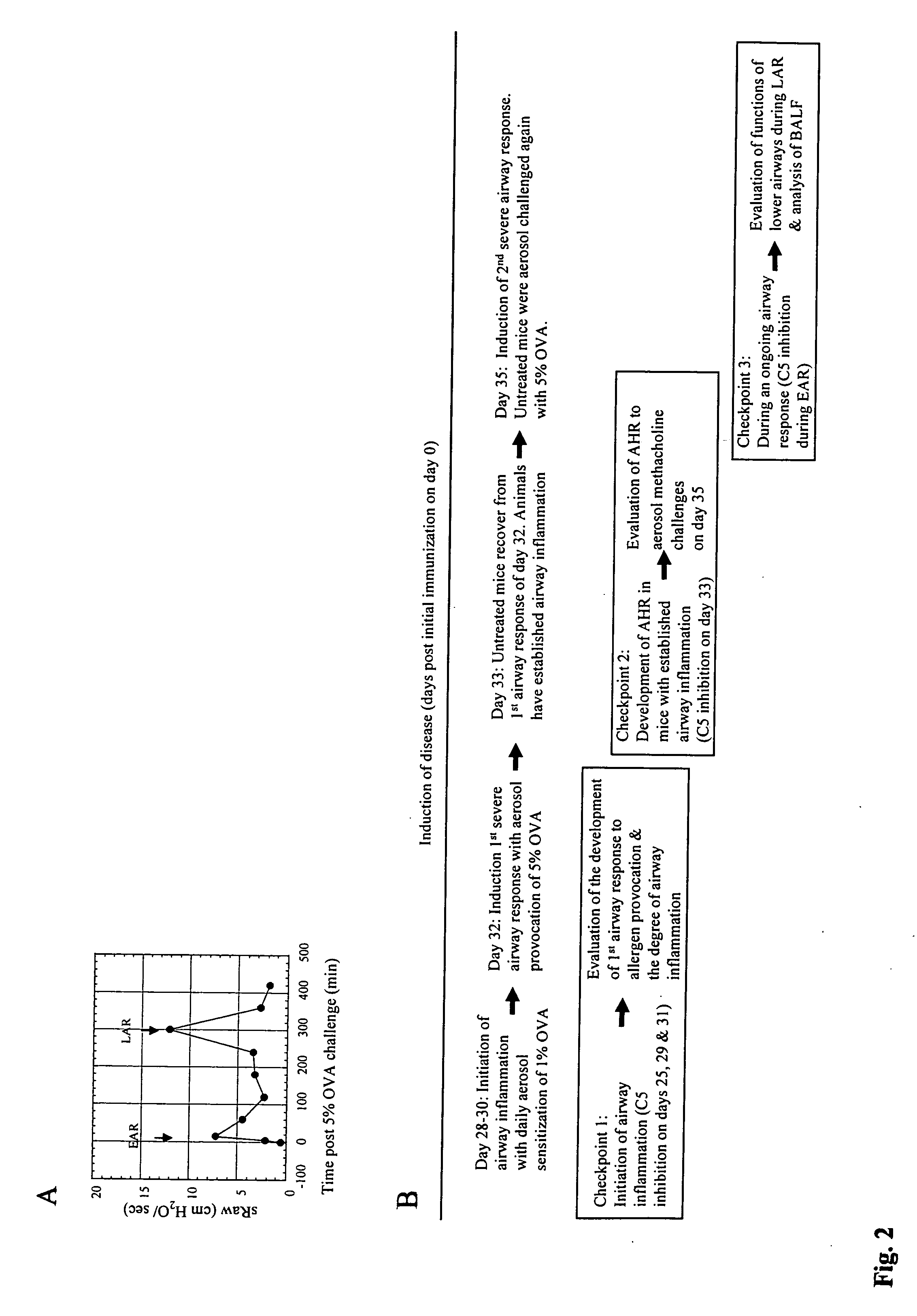
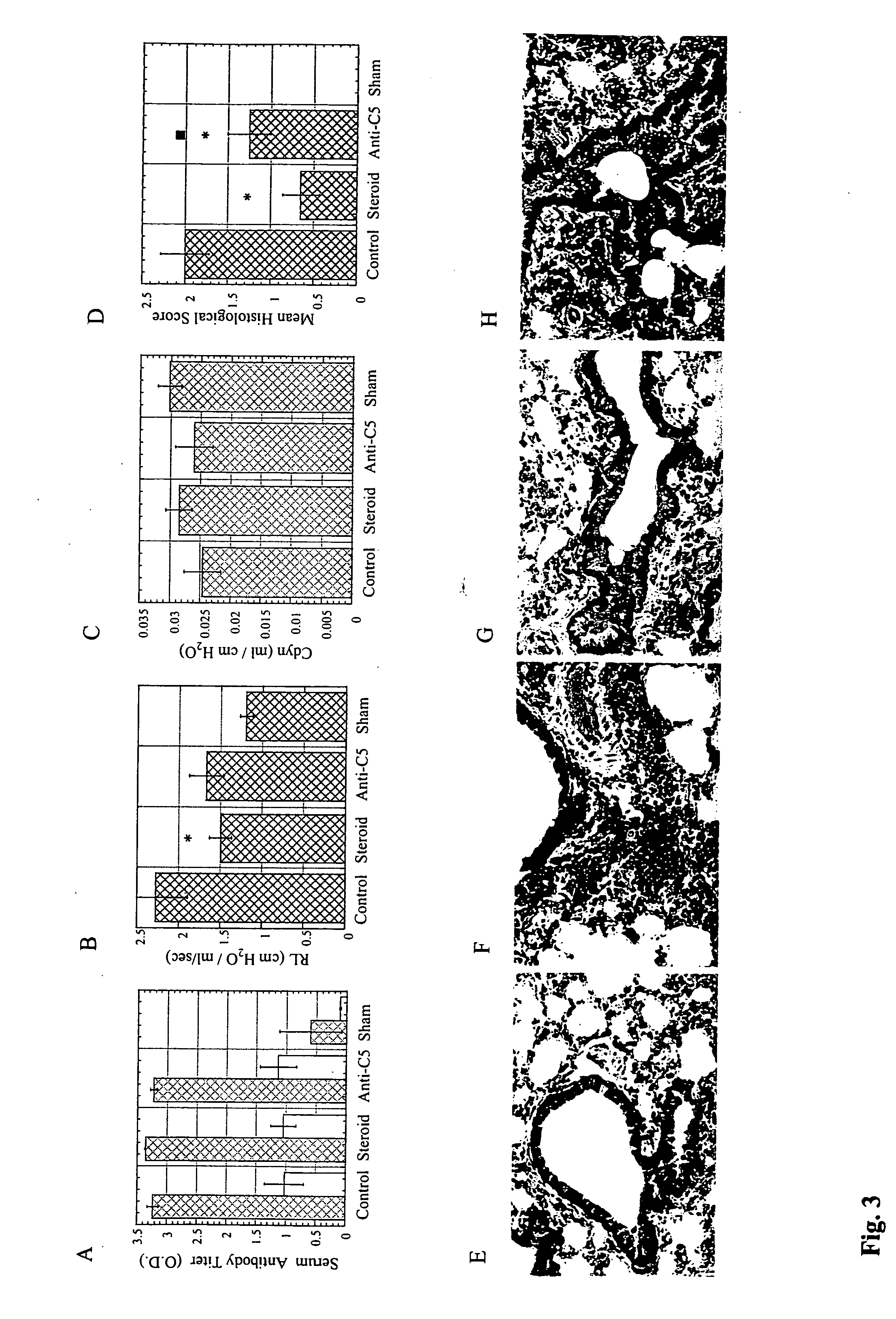
[0115] Animals: Male C5s BALB / cByJ, C57BL / 6J mice and C5d B10D2oSn / J, AKR / J and SWR / J mice were purchased from the Jackson Laboratory and housed in a pathogen free facility. In addition to serum C5b-9 mediated hemolysis, the C5 genotype was further confirmed by PCR performed on tail DNA using a pair of primers (5′-CAC GAT AAT GGG AGT CAT CTG GG-3′ (SEQ ID NO:1) and 5′-AAG TTG GAG TGT GGT CTT TGG GCC-3′ (SEQ ID NO:2) that amplify a 280-bp DNA fragment from both C5s and C5d DNA. This fragment encodes a HindIII site that is selectively destroyed by a mutation in the C5 gene, such that HindIII (New England BioLabs, MA) digestion selectively cleaves the C5s but not the C5d PCR products into 150 and 130-bp fragments. All animal protocols were reviewed by Institutional Committee and were in accordance with NIH guidelines.
[0116] Reagents: Anti-mouse C5 mAb (BB5.1) (Wang et ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com