Phase variation test method for salmonella H antigen
A Salmonella, test method technology, applied in the direction of resistance to vector-borne diseases, biological testing, material testing products, etc., can solve the problems of serum agglutination particles confusion, hinder H antigenic recovery, interfere with agglutination results, etc., to achieve easy observation and judgment. , Eliminate antagonism, obvious effect of agglutination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
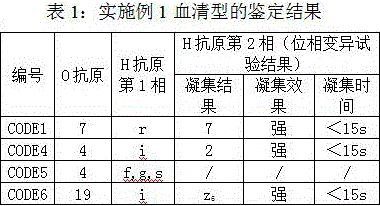
[0015] In 2015, the State Food and Drug Administration conducted a blind sample assessment (Salmonella inspection) of food safety sampling inspection and inspection agencies, with a total of 10 samples, coded as CODE1~10. If Salmonella is detected, the isolated strains need to be serotyped. After testing, 4 samples of CODE1, CODE4, CODE5, and CODE6 among the 10 samples were positive for Salmonella. Then follow the steps below to carry out the follow-up test:
[0016] a. The serum agglutination reaction of O antigen was carried out for the 4 Salmonella strains to be tested, and the H factors of phase 1 and phase 2 were checked in turn according to the grouping of O antigen. The identification results were CODE1 (O 7: H r), CODE4 (O 4: H i), CODE5 (O 4: H f, g, s), CODE6 (O 19: H i), among which the strain to be tested for CODE5 is a monophasic bacteria, so three samples of CODE1, CODE4, and CODE6 were determined The Salmonella positive strains were the target strains.
[0017...
Embodiment 2
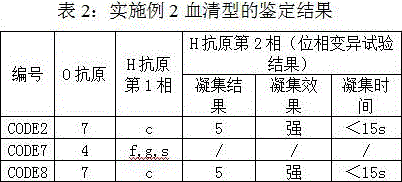
[0025] In 2016, the State Food and Drug Administration conducted the blind sample assessment of food safety sampling inspection agencies at the same level. Among them, a total of 10 samples were tested for Salmonella, coded as CODE1~10, and reported that Salmonella was detected or not. Three samples of CODE2, CODE7, and CODE8 were tested positive for Salmonella among the 10 samples. Then follow the steps below to carry out the follow-up test:
[0026] a. Serum agglutination reaction of O antigen for the three Salmonella strains to be tested, and check the H factors of phase 1 and phase 2 in turn according to the grouping of O antigen, and the test results are CODE2 (O 7: H c); CODE7 (O 4: H f, g, s), CODE8 (O 7: Hc), where the strain to be tested for CODE7 is a single-phase bacteria, so the positive strains of Salmonella in two samples of CODE2 and CODE8 are determined as the target strain.
[0027] b. Take the serum of known phase H factor into sterile nutrient broth (the vo...
Embodiment 3
[0035] A verification experiment of serotyping was performed on a strain of Salmonella typhimurium (strain number: CGMCC1.1174). After the strain is activated, follow the steps below to carry out follow-up tests:
[0036] a. Serum agglutination of the Salmonella strain to be tested is carried out with the O antigen, and the H factors of phase 1 and phase 2 are checked sequentially according to the grouping of the O antigen. The identification result is O 4: H i, so it is determined to be the target strain;
[0037] b. Take the serum of known phase H factor into sterile nutrient broth (the volume ratio of serum to nutrient broth is 1:800), mix well, inoculate the target strain, and incubate at 36°C±1°C for 24 hours to obtain Cultures;
[0038] c. Transfer the culture to a sterile centrifuge tube, centrifuge at 8000 r / min for 5 min, discard the supernatant, and keep the bacterial pellet at the bottom of the centrifuge tube;
[0039] d. Add sterile normal saline (the volume ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com